A METHOD FOR THE AQUEOUS DEACIDIFICATION OF OXIDIZED PAPERJOHN BOGAARD, HANNAH R. MORRIS, & PAUL M. WHITMORE
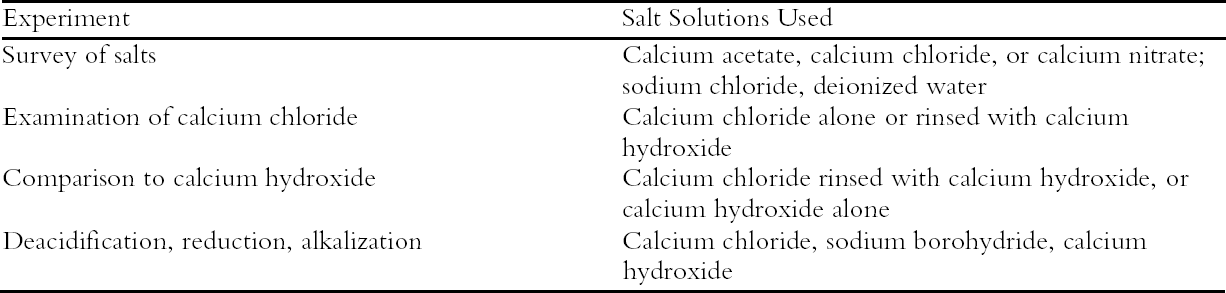
ABSTRACT—Concentrated aqueous solutions of pH-neutral salts were evaluated for their effectiveness at deacidifying photo-oxidized filter paper. Short immersions in these solutions successfully neutralized acidic paper sheets. However, subsequent rinses to remove excess salt were necessary to prevent yellowing upon thermal aging. Sheets treated with concentrated solutions of calcium chloride showed no significant chemical damage from the treatment and deteriorated more slowly during thermal aging compared with untreated sheets. Sample papers that were given a dilute alkaline rinse after the concentrated salt treatment degraded less upon thermal aging than papers treated with either solution alone. This method of neutral deacidification was also evaluated for use prior to chemical reduction of oxidized paper with sodium borohydride. A treatment procedure involving steps of deacidification, chemical reduction, and alkalization was found to greatly stabilize photo-oxidized papers against thermal aging, as well as to produce a bleaching effect. TITRE—Une m�thode pour la d�sacidification aqueuse des papiers oxyd�s. R�SUM�—Des solut�s concentr�s de sels neutres ont �t� �valu�s pour leur efficacit� � d�sacidifier du papier filtre photo-oxyd�. Des immersions de courte dur�e dans ces solutions ont neutralis� les feuilles de papier acide avec succ�s. Cependant, des rin�ages successifs furent n�cessaires pour �liminer les exc�dents de sel afin d'emp�cher le jaunissement caus� par le vieillissement thermique. Les feuilles de papier trait�es avec des solutions concentr�es de chlorure de calcium n'ont montr� aucun dommage chimique significatif caus� par le traitement et ont d�t�rior� plus lentement pendant le vieillissement thermique par rapport aux feuilles non trait�es. Les �chantillons de papier qui ont re�u un rin�age alcalin dilu� apr�s le traitement en solution concentr�e se sont moins d�t�rior�s pendant le vieil-lissement thermique que les �chantillons trait�s avec seulement l'une ou l'autre solution. L'usage de cette m�thode de d�sacidification neutre avant la r�duction chimique du papier oxyd� avec le borohydrure de sodium a �t� �galement �valu�. Un proc�d� de traite-ment comprenant des �tapes successives de d�sacidi-fication, r�duction chimique et d'alcalisation s'est av�r� tr�s efficace pour stabiliser consid�rablement les papiers photo-oxyd�s contre le vieillissement ther-mique; il a aussi produit un effet de blanchiment. TITULO—Un m�todo para la desacidificaci�n acuosa de papel oxidado. RESUMEN—Se evalu� la efectividad de las soluciones acuosas concentradas de sales neutras para la desacidificaci�n de papel de filtro foto-oxidado. Las inmersiones de corta duraci�n de estas soluciones neutralizaron con �xito las hojas de papel �cido. Sin embargo, fueron necesarios enjuagues subsiguientes para remover los excesos de sal y prevenir el amarillamiento luego del envejecimiento por calor. En comparaci�n con las hojas no tratadas, las tratadas con soluciones concentradas de cloruro de calcio no mostraron da�o qu�mico significativo por el tratamiento, y se deterioraron m�s lentamente durante el envejecimiento por calor. Los papeles de muestra, a los que se les dio un enjuague alcalino despu�s del tratamiento concentrado con sales, se degradaron menos por envejecimiento por calor que los papeles tratados con cualquiera de estas soluciones solamente. Este m�todo de desacidificaci�n neutra tambi�n fue evaluado para su uso previo a la reducci�n qu�mica de papel oxidado con borohidruro de sodio. Se encontr� que el proceso de tratamiento que involucra pasos de desacidificaci�n, reducci�n qu�mica, y alcalinizaci�n, estabiliza en gran medida los papeles foto-oxidados en contra del envejecimiento por calor a la vez que produce un efecto blanqueador. T�TULO—Um M�todo Para Deacidifica��o Aquosa de Papel Oxidado. RESUMO—Solu��es concen-tradas de sais neutros foram avaliadas quanto 'a efici�ncia na deacidifica��o de papel-filtro foto-oxidado. Imers�es curtas nestas solu��es neutralizaram com sucesso folhas de papel �cido. Todavia, foram necess�rios banhos subsq�entes para remover o excesso de sais para prevenir o amarela-mento ap�s envelhecimento por calor. Folhas tratadas com solu��es concentradas de cloreto de c�lcio n�o mostraram nenhum dano qu�mico causado pelo trata-mento e se deterioraram mais lentamente durante o envelhecimento por calor, comparando-se com as folhas n�o tratadas. Amostras de pap�is, que foram submetidas a banhos alcalinos dilu�dos ap�s o trata-mento com sais concentrados, sofreram menos degrada��o durante o envelhecimento por calor do que os pap�is tratados com cada uma das solu��es em separado. Este m�todo de deacidifica��o neutra foi tamb�m avaliado para o uso antes da redu��o qu�mica de pap�is oxidados com borohidrato de s�dio. Um 1 INTRODUCTIONTreatment of acidic, oxidized paper can pose a difficult problem for conservators. The oxidation of cellulose has been found to increase the rate of hydrolytic degradation upon thermal aging (Whitmore and Bogaard 1995), magnifying the need for deacidifica-tion. But immersion of oxidized papers in alkaline solutions may cause some breakage of the cellulose chains near the oxidation sites in conditions as mild as pH 9 (Luetzow and Theander 1974). Treatment with dilute alkaline solutions may reduce this risk; however, photo-oxidized sheets have been found to be harder to stabilize than unoxidized ones (Bogaard and Whitmore 2001). It can be difficult to gauge the extent to which a paper has been oxidized unless such factors as the composition of the pulp furnish and the history of light exposure and bleaching are well known. Lacking this information, it is difficult to assess the risks of damage from alkaline treatments. Chemical reduction of oxidized paper with sodium borohydride can stabilize the sheet (Tang 1986), as well as make it suitable for alkaline deacidi-fication. However, solutions of sodium borohydride can be highly alkaline, and some degradation of the paper may occur during treatment until the reduction is complete. The borohydride molecule also decomposes upon contact with an acidic paper and liberates hydrogen gas, sometimes with a very vigorous bubbling action that can damage friable ink or media, and this effect slows the reduction process (Varshney and Luner 1961). Neutralization of the paper prior to reduction is recommended (Burgess et al. 1989). So the conundrum a paper conservator faces when treating acidic, oxidized objects is that the best treatments for imparting long-term stability may themselves cause some immediate degradation, the extent of which may be difficult to gauge ahead of time. A method of neutralization that reduces these risks is desirable. In the study described here, a technique to address this problem was examined that utilizes the affinity of the cellulose polymer for behaving as an ion-exchange resin and attempts to deacidify paper by treatment with a concentrated neutral salt solution rather than an alkaline solution. When a paper sheet is immersed in an aqueous salt solution, ion exchange occurs as the acidic hydrogen ions in the cellulosic fibers exchange with the cations of the salt solution until equilibrium is reached. The rate of this process is controlled by diffusion into and out of the fibers, a process that is driven by the difference in ion concentrations between the fiber and the solution. When the concentration gradient is small, such as with deionized water, weakly alkaline solutions, or dilute salt solutions, the displacement of acids from the fibers proceeds very slowly. By using a stronger solution to increase the concentration gradient, the process can be accelerated (Helfferich 1962). This is the principle behind the modified pH test for paper suggested by Scallan (1990), in which the sample is soaked in a 0.1 molar solution of sodium chloride, rather than deionized water, to reach equilibrium faster and obtain a more accurate pH reading. The equilibrium attained by soaking an acidic paper in a concentrated neutral salt solution may not remove all the acids from the paper fibers; alkalinity may be needed to neutralize the solution and drive the ion exchange to completion. Treatment with concentrated neutral salt solutions may work best as a first step in a sequence of treatments. This study explored the possible use of concentrated neutral salt solutions in deacidification treatments for acidic, oxidized papers and assessed the benefits and risks of this technique. A variety of salt solutions were evaluated for their effectiveness at removing acids from photo-oxidized paper sheets. Calcium salts were focused on, due to the strong affinity of calcium ions for cellulose and their binding power to carboxylic acid groups on the cellulose chain (Ohlsson and Rydin 1975). Calcium chloride was found to effectively neutralize oxidized paper and was studied in greater detail as a possible first step in a conservation treatment. Since calcium ions carry a double positive charge, they are likely to bear another anion, generally from the original salt, when bound to a carboxylate, so an alkaline rinse was used to exchange the residual chloride ion with an hydroxyl ion as well as to complete the deacidification and leave an alkaline reserve. Comparisons were made between treatments with calcium chloride solutions alone, calcium chloride treatment followed by an alkaline rinse, and an alkaline rinse alone to determine the most effective procedure for slowing paper degradation. Concentrated calcium chloride solutions were also evaluated for use as a neutralization step prior to chemical reduction with sodium borohydride. 2 EXPERIMENTAL APPROACHThe focus of these experiments was to evaluate the effects of concentrated salt solutions on oxidized cellulose; their effect on other paper components or on ink or other media was not examined. To follow chemical changes in the samples, tests were performed on a pure cotton cellulose filter paper, Whatman no. 42, that was oxidized prior to treatment by exposure to ultraviolet-A lamps (emitted wavelengths 320–400 nm). Exposure times were different for each experiment and ranged from 70 to 168 hours. Some of the exposed sheets were also thermally aged at 90�C and 50% RH prior to treatment. The photo-oxidation and accelerated aging of the filter paper was an attempt to simulate the deteriorated papers encountered by conservators. Chemical tests of the treated sample sheets were performed before and after accelerated thermal aging. The details of these tests are described in appendix 1 and will be briefly summarized here. The viscosity of cellulose solutions in cupriethylenedi-amine was measured to calculate the degree of polymerization (DP) of the cellulose chains. From the D P, the concentration of scissions, or new breaks in the chains, was calculated. Graphs of the degradation of the samples upon aging have been drawn in terms of the scissions, which illustrate more clearly than the DP the progress of the degradative reactions. The concentrations of carbonyl and carboxyl oxidation groups were measured using colorimetric methods: the hydrazine technique for the carbonyl group and the methylene blue method for the carboxyls. The units for the concentrations of oxidation groups and scissions have been standardized to mmol/100 g to allow for easy comparison. As noted in an earlier paper (Whitmore and Bogaard 1994), the changes in oxidation group content can be compared to the scissions produced to discern the dominant degradation pathway. If these numbers are approximately equal, acid-catalyzed hydrolysis is indicated. Significantly higher oxidation changes of at least two to three times more than the scissions indicate an oxidative process. The pH was measured using the standard cold extract method, usually with the modification of soaking in a solution of sodium chloride (Scallan 1990) rather than deionized water. The brightness of the sheets was tracked and is expressed in terms of % reflectance (%R) at 460 nm, which indicates yellowing of the paper by a decrease in reflectance. No other reflectance changes besides the decrease at low wavelengths (i.e., no other color changes besides yellowing) were observed. The concentrated salt solutions were 0.1 M and had a pH that ranged from 6.7 to 7.9; the concentrated solutions of calcium chloride ranged in pH from 7.2 to 7.4. The dilute calcium hydroxide solutions were prepared from 40-fold dilutions of a saturated calcium hydroxide solution, approximately 0.4 mM in concentration, with a pH around 10. For all the treatments, paper was immersed in the designated solution for only about 15-30 minutes; when more than one bath was used, samples were immersed successively without drying. Four sets of experiments were performed on the photo-oxidized filter paper, and they are summarized in table 1. First was a survey of the deacidifying effects of concentrated solutions of four different neutral or slightly alkaline salts (calcium acetate, calcium chloride, calcium nitrate, and sodium chloride) compared to deionized water. The next experiment examined the effects of concentrated calcium chloride solutions, with and without a following rinse of dilute (approx. 0.4 mM) calcium hydroxide. The third experiment employed photo-oxidized sheets that were thermally aged prior to treatment to create more degraded, acidic samples. A comparison was
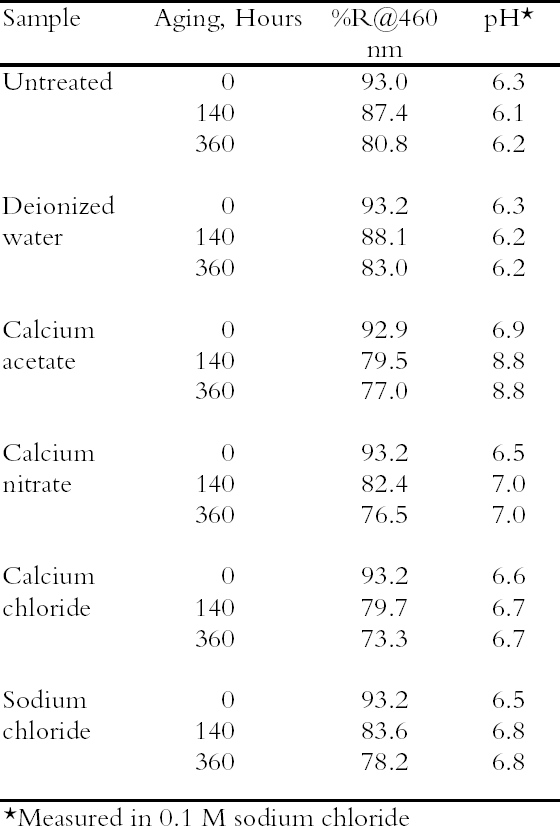
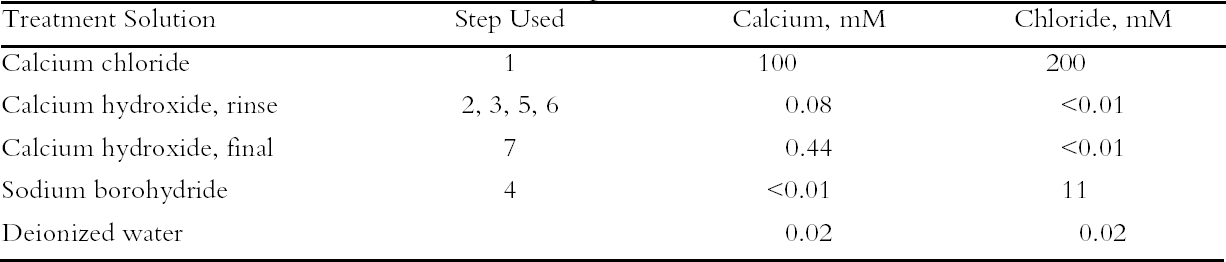
The last experiment demonstrated a series of treatment steps that included a chemical reduction to suggest a possible way that a paper conservator could integrate the use of concentrated salt solutions into a treatment regimen. To simulate the aged, degraded papers that are difficult to treat, sample sheets were selected that had been aging at room temperature for more than seven years after exposure to near-ultraviolet light. The samples were treated through a series of seven steps: (1) immersion in the concentrated calcium chloride solution; (2) and (3) two successive rinses in an extremely dilute solution of calcium hydroxide (approx. 0.08 mM, a 200-fold dilution of the saturated solution and about five times more dilute than the calcium hydroxide solution used in the previous experiment); (4) immersion in a bath of 0.01 M sodium borohydride to chemically reduce the cellulose; (5) and (6) two more rinses with the 0.08 mM calcium hydroxide solution; and (7) a final soak in 0.4 mM calcium hydroxide solution. The solution of sodium borohydride used here was about 25 times more dilute than the solution used in our previous work (Bogaard and Whitmore 2001); both strengths are within the range known to be used by conservators (Tang 1986). To follow the uptake of calcium by the sample sheets, as well as to ensure that the chloride ions were rinsed out, contents of calcium and chloride ions in the sample sheets were measured using ion-selective electrodes attached to the pH meter. 3 RESULTS AND DISCUSSION3.1 SURVEY OF CONCENTRATED SALTSPhoto-oxidized papers were immersed in concentrated (0.1 M) solutions of four different salts as well as deionized water, and the unrinsed sheets were thermally aged. The results are summarized in table 2; in this case only pH and brightness were tracked. The oxidized paper was only mildly acidic (pH = 6.3), and all the salt solution treatments raised the pH almost to neutrality, while the water treatment did not change the pH. Upon thermal aging, the pH of the treated sheets rose. This effect was small for three of the salts but much higher for the calcium acetate. In this case, it may be that, at the high temperature and humidity of thermal aging, the excess salt decomposed to form acetic acid that then vaporized, leaving an alkaline residue. It was decided not to examine this salt further. The reflectance data show greater yellowing of the salt-containing sheets upon thermal aging compared with the untreated or water-treated samples. Yellowing has been seen frequently with magnesium salt treatments (Bansa 1998), but it has been found in fewer instances with calcium salts (Hey 1979; Tang 1981). It is not clear what causes this effect or to what extent it would occur if the samples were held at room temperature. Nonetheless the effect is undesirable, and ways to minimize it were explored in the following experiments. All the salts surveyed deacidified the paper samples to a satisfactory degree, and all the treated sheets yellowed upon thermal aging. Since there was no substantial difference between them, calcium chloride was chosen for further examination as a possible conservation treatment, due primarily to its inert nature and high solubility.
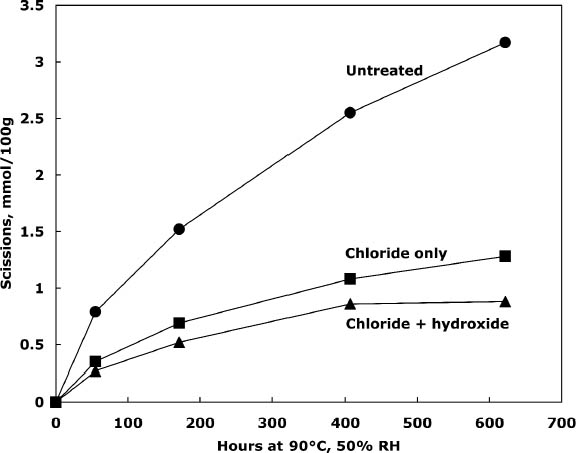
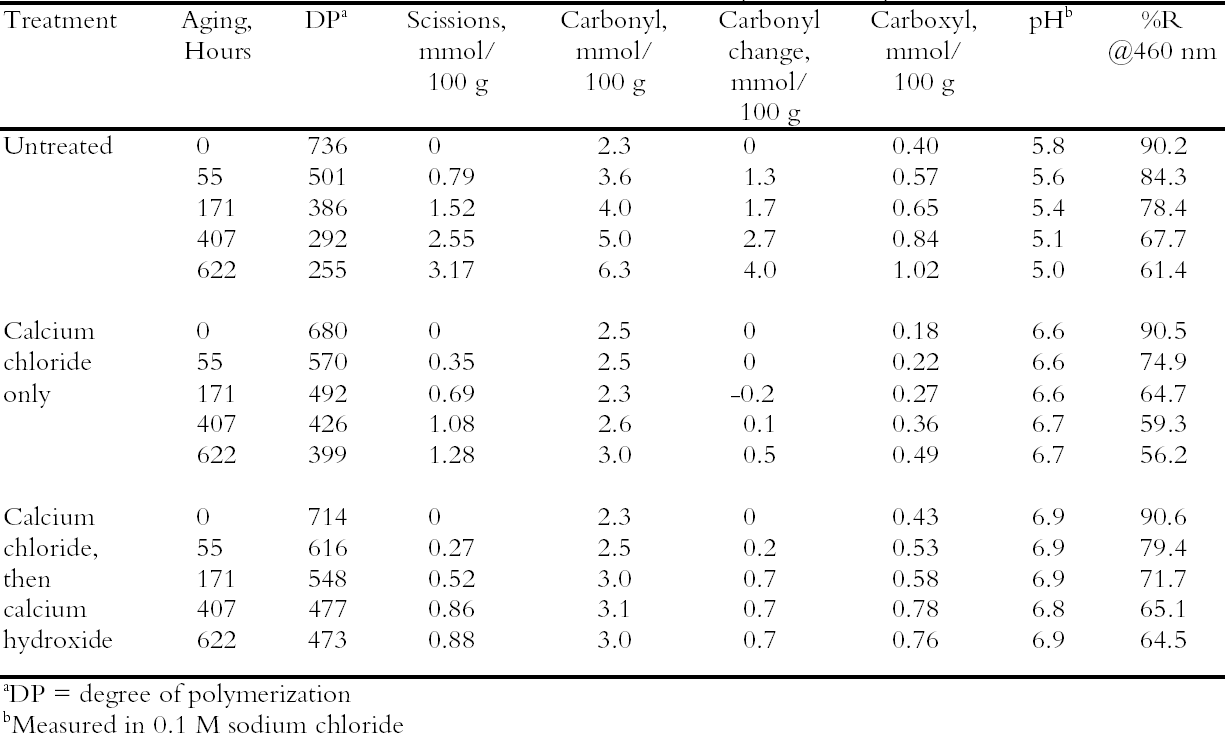
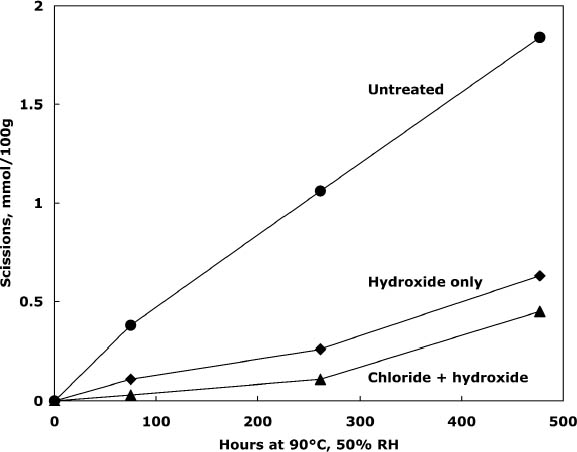
3.2 TREATMENT WITH CALCIUM CHLORIDE ALONE OR WITH CALCIUM HYDROXIDEPhoto-oxidized sheets treated with calcium chloride alone were compared with samples that were treated and then rinsed once with dilute calcium hydroxide to remove excess salt and give an alkaline reserve. The results of the treatment and subsequent thermal aging are collected in table 3. The treatment with chloride only raised the pH by almost a full unit before thermal aging, while the two-step treatment raised it even more. There was a slight reduction in DP after both treatments, which is not thought to be significant. The carbonyl group content was virtually unchanged for the two-step treated sample and slightly higher for the chloride-only treatment (probably due to its lower DP). The carboxyl content was also virtually unchanged for the two-step sample. The reduction of measured carboxyl groups with the chloride-only treatment was probably due to interference with the methylene blue test by the high amount of residual calcium in the sheet rather than an actual loss of these groups (Davidson 1948). The thermal aging results are graphed in figure 1 in terms of the cellulose scissions, or chain breaks, versus the hours of aging. The untreated paper deteriorated quickly, while the treatments greatly reduced this rate. The treatment with chloride only slowed the degradation by about two to three times, while the two-step treatment slowed it about three to four times. Referring back to table 3, the untreated sample showed a steady drop in pH over the aging period, while the carboxyl content grew significantly. For the two-step samples, the pH was unchanged, despite the increase in carboxyls,
Comparison of the scissions with the change in carbonyl content shows an approximately equal ratio, indicating that the degradation was primarily due to acid hydrolysis, as expected when paper is thermally aged. It is unusual that the samples treated with chloride only did not seem to change in their carbonyl content at first despite their deterioration; every break in the cellulose chain should produce a new reducing end that is measured as a carbonyl group. It is not clear if the chain-breaking chemistry is altered for those samples that have a high amount of residual salt or if the excess salt is interfering with the carbonyl measurement in some way. The reflectance measurements show that the treated samples yellowed faster initially than the untreated paper, even though the treated papers were chemically less degraded. However, the rate of darkening for these sheets gradually slowed as the aging progressed, unlike the untreated papers, which continued to darken steadily. The rinse of the paper after the concentrated salt treatment seemed to improve the darkening behavior, so more thorough rinsing was performed in the next experiment to see if the problem could be eliminated. 3.3 COMPARISON OF THE TWO-STEP TREATMENT TO CALCIUM HYDROXIDE ALONEPhoto-oxidized sheets were thermally aged before treatment to create more acidic, deteriorated samples that would pose a more difficult challenge to treat. After treatment with concentrated calcium chloride, the sample papers were rinsed twice with dilute calcium hydroxide to thoroughly remove excess salt and leave an alkaline reserve. Comparison was made with samples that were simply rinsed twice with dilute calcium hydroxide. The results of treatment and subsequent thermal aging are contained in table 4. The untreated samples had a pH of 5.6, while the pH of the treated sheets was raised to 6.8 in both cases. Both treatments showed a very slight reduction in DP coupled with a slight increase in carbonyl groups. There was virtually no change in the carboxyl content; rinsing after the concentrated salt treatment apparently eliminated the interference with the methylene blue test. The degradation of the samples upon thermal aging is shown in figure 2. Both treatments retarded the deterioration with the two-step treatment, slowing the degradation rate by four to five times and the hydroxide-only treatment, slowing it by about three to four times. These results were reflected in
The results of these last two experiments show that combining the two treatments for neutralization and alkalization worked better than either treatment alone in slowing degradation upon thermal aging, and they caused virtually no chemical damage. The concentrated calcium chloride solution seems to quickly draw out a significant amount of acidity from paper fibers, leaving the calcium hydroxide treatment better able to complete the deacidification process and create an alkaline reserve. However, it may also be desirable to stabilize or bleach oxidized papers with a reduction treatment. The drawbacks of using sodium borohydride to reduce the oxidation have been noted earlier; neutralizing the paper first should make this treatment more efficient and lower the risk of damage from both the alkalinity and the gas bubbles. A treatment sequence of neutralization of an acidic, oxidized paper with the concentrated salt solution, followed by reduction of the oxidation and then an alkaline rinse, should decrease the risks of these treatments while enhancing their beneficial effects. In the next section this sequence is demonstrated. 3.4 NEUTRALIZATION, REDUCTION, AND ALKALIZATIONThe oxidized, naturally aged paper was given a seven-step sequential treatment that included neutralization with concentrated calcium chloride solution, two rinsing steps, reduction with sodium borohy-dride solution, two more rinsing steps, and final deacidification with dilute (0.4 mM) calcium hydroxide solution. During the immersion of sample papers in the borohydride reduction step, it was observed that only a small amount of tiny bubbles formed on the neutralized sheet, which indicated that the decomposition of sodium borohydride was minimal. This result created a safer situation for the paper with regard to the bubbling and presumably a more efficient reduction treatment.
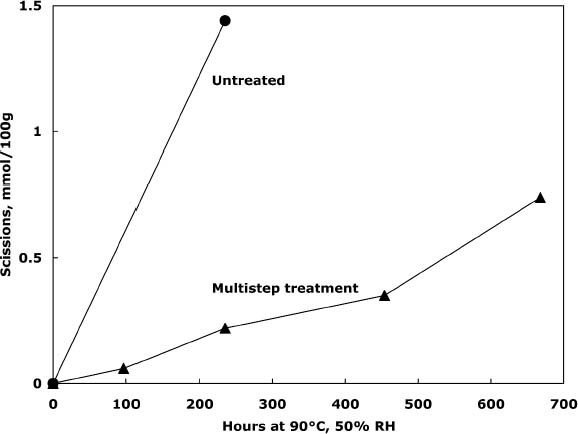
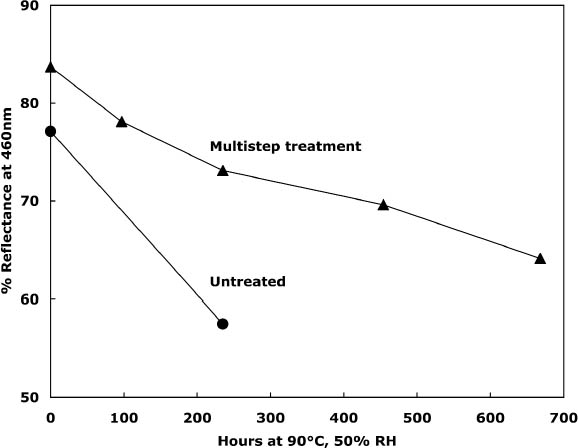
To characterize the treatment solutions more fully, the concentrations of calcium and chloride ions were measured using ion-selective electrodes. The data, presented in table 5, show the concentrations for the calcium chloride solution that were expected. The dilute calcium hydroxide solution is less concentrated, at 0.44 mM, than the estimation of 0.63 mM that was based on dilution of a saturated solution. Despite excess calcium hydroxide in the solution, full saturation apparently was not achieved. The solution of sodium borohydride had a surprisingly high amount of chloride impurity. The deionized water concentrations are presented for comparison and, as expected, had only trace amounts of the ions. The results of the chemical tests after treatment and thermal aging are found in table 6. The untreated sheet was fairly degraded with a pH of 4.5. The multi-step treatment raised the pH by almost two units to 6.4, while the DP was virtually unchanged. The lower value of the carbonyl content in the treated sheet roughly corresponded to reduction of all the oxidation groups on the cellulose chain. Calculations suggest that the remaining carbonyls may be the end groups of the chains, which are slower to react (Head 1955). The degradation of the samples upon thermal aging is shown in figure 3. (Due to the limited amount of these samples, only one untreated sheet was included in thermal aging for comparison.) The treated samples degraded about seven times more The brightness measurements (%R) indicate an increase in the reflectance of the sheets after treatment; this bleaching should be due to the sodium borohydride treatment (Tang 1986). The yellowing upon thermal aging is graphed in figure 4, and it can be seen that the rate of discoloration was much lower for the treated sheets than for the untreated. Measurements of the calcium and chloride ion contents in the treated sheets are included in table 6. The measurements are presented in units of mmol/100 g for comparison to the oxidation tests. (For comparison with other published work, these amounts are equivalent to 250–280 ppm of calcium in the treated sheets.) The treatments raised the calcium content to 0.68 mmol/100 g, which is a little lower than the carboxyl content of 1.00 mmol/100 g. The calcium ion is capable of binding with two carboxyl groups if they are in close proximity, so it is not known if all the carboxyls have been converted to the salt or if some remain in the acid form. However, the pH measurements imply that this paper is not fully neutralized. The chloride content was lowered slightly by this treatment sequence. The thorough rinsing with calcium hydroxide seemed to remove any chloride ions introduced by the concentrated salt treatment. With thermal aging the chloride content dropped in both the treated and untreated sheets. It is not clear if the high temperature and humidity conditions caused the ion to bind to something in the paper, forming an insoluble salt, or to react in another, unknown way. The amount of calcium in the sheets remained about the same upon aging; the variation between samples is probably due to differences in pickup during treatment.
4 CONCLUSIONSA method for the aqueous deacidification of paper by immersion in a concentrated solution of a neutral salt has been examined. This technique increases the rate at which acidic ions diffuse out of the paper fibers in an ion-exchange process. Papers are quickly neutralized; however, no alkaline reserve is left by this treatment. Photo-oxidized filter papers treated with this technique showed little or no chemical damage, and when subjected to thermal aging they were stabilized compared to untreated sheets. However, if the salt is not rinsed out of the sheet after treatment, there may be some yellowing. Following the concentrated salt solution with a dilute alkaline treatment to rinse out the salt and leave an alkaline reserve stabilized the sheets against thermal aging more than either treatment alone and mitigated the excess yellowing. Treatment of acidic, oxidized paper with a concentrated neutral salt solution may also be appropriate as a first step before chemical reduction. Photo-oxidized paper was greatly stabilized to thermal-aging by a multistep treatment that involved neutralizing the paper with the concentrated salt solution, rinsing out the salt, then reducing the oxidation by treatment with sodium borohydride, and washing with an alkaline solution. By neutralizing the paper first, the treatment with sodium borohydride was made safer and more efficient by slowing its decomposition and subsequent release of hydrogen gas bubbles. The salt examined most closely in this study was calcium chloride. This salt should be appropriate for most circumstances.1 However, it is not recommended for papers that contain a significant amount of silver or lead, due to the possibility of forming insoluble chloride salts. A survey of other salts showed that concentrated solutions of many neutral salts could also be effective. This study examined the effects of concentrated salt treatments on cellulose alone and did not look at interactions with other paper components or with ink or media. More research needs to be done to assess the effects of these treatments on commercial paper as well as on printing and artistic materials. And, of course, for heavily sized or coated papers that do not wet easily, a completely different strategy to remove acidity and stabilize the sheet must be devised. Nonetheless, for papers that are sensitive to alkalinity and that can be subjected to aqueous treatments, this technique could be a safe, effective, and rapid method for removing acids from them and stabilizing them. APPENDIXAPPENDIX 1: EXPERIMENTAL DETAILSWhatman no. 42 filter paper, a pure cotton linter paper, was first photo-oxidized by exposure to ultra-violet-A lamps, specifically the Q-Panel UVA-351 bulbs. During exposure the samples were held 4 in. from the light source, producing approximately 6 mW/cm2 intensity in the 300–400 nm wavelength range. The paper studied in the final experiment was also Whatman no. 42 that had been exposed to UV-A Viscometric degrees of polymerization of single samples of each test sheet were determined by the standard method (ASTM 1962) in a solution of 0.5M cupriethylenediamine, following overnight (approximately 18-hour) treatment in unbuffered 0.01M sodium borohydride to stabilize the alkali-sensitive linkages. The concentration of chain scissions is derived from this DP by first converting to the number-average DP and then calculating the concentration of chains, which is expressed in the same units as the carbonyl and carboxyl analyses (mmol/100 g). The difference in the concentration of chains between unaged and aged samples gives the concentration of scissions that have occurred. For a more detailed explanation of these calculations, see Whitmore and Bogaard (1994). The precision of the viscosity measurements (reported by ASTM to be about 3%) is such that changes in scissions of more than 0.1 unit are considered significant. Carbonyl contents were measured using the hydrazine method (Albertsson and Samuelson 1962). Reported values are the average of duplicate measurements made on two replicate samples taken from the same test sheet. This procedure serves to increase the precision of this technique to approximately plus or minus 10% of the value. Carboxyl contents were assayed using the standard methylene blue test (ASTM 1963). Reported values are results for single analyses, which are said by ASTM to have a precision of about 2-8%, with the higher values being slightly more precise than the lower. Neither the hydrazine nor methylene blue tests are able to detect oxidation on soluble fragments of the cellulose polymer. Measurements of the cold extract pH were made using the standard method (TAPPI 1988) with the modification of using 0.1M sodium chloride, except when the ion contents were also being measured; in this case deionized water was used to soak the samples. Precision of these results is about 0.1 pH unit. Measurements of the calcium and chloride ion contents of the paper samples were made with ion-selective electrodes using the ratio of 0.5 g paper to 50 ml deionized water. Since the samples were run concurrently, the same ionic strength adjustor, a solution of sodium nitrate, was used for both. First the chloride contents of all the samples were measured. Then the paper/water slurry was acidified to pH 3.5 using drops of 0.1 N hydrochloric acid; this procedure took from 0.2 to 0.4 ml. The acidification ensured that the calcium ions were fully released from the paper fibers prior to their measurement, which followed. Treatment solutions were prepared from degassed water that had been deionized in a reverse osmosis system. The solutions used in the final, multistep treatment were made with deionized water that was put through an ion-exchange column to remove trace minerals (primarily sodium chloride) that are retained after the reverse osmosis purification. The contents of calcium and chloride ions in the treatment solutions were measured with ion-selective electrodes, using a sodium nitrate ionic strength adjuster. Since a reducing solution can interfere with measurement by the chloride electrode, the reducing power of the sodium borohydride solution was quenched by dropwise addition of a solution of potassium permanganate prior to measuring its chloride content. Reflectance spectra of single sheets backed with Millipore were measured on a MacBeth ColorEye 7000 spectrophotometer. Brightness (%R at 460 nm) is reported. The reflectance changes at this wavelength track the yellowing of the papers (decreased reflectance at blue wavelengths), and no other color changes besides yellowing were observed. NOTES1. Calcium chloride may seem an unusual choice for a conservation treatment, and conservators may be understandably wary of introducing chlorine into their papers. However, chlorine is a common impurity found in most papers, especially those that have been bleached. Even ashless Whatman filter paper shows chlorine as the trace element found in the greatest abundance (Whatman Inc. 1983). It should be noted that there is a vast difference in reactivity between the different forms of chlorine. The chlorideion (Cl ) generally forms inert salts that are found in common foodstuffs, road treatments, etc. By contrast, the oxygen-containing chlorite (ClO2-) or hypochlorite (ClO) ions are highly corrosive and are used primarily for bleaching and disinfecting.Chloride salts of different metals, such as iron, copper, and magnesium, are all highly soluble in water, so if the chloride ion reacts with another trace metal in the paper during treatment, it should be ACKNOWLEDGEMENTSThis work was performed at the Art Conservation Research Center (formerly the Research Center on the Materials of the Artist and Conservator) at Carnegie Mellon University. The financial support of the Andrew W. Mellon Foundation is gratefully acknowledged. John Bogaard thanks Dr. Robert L. Feller, emeritus director of the Center, for helpful discussions of this paper. Parts of this paper were presented at the American Institute for Conservation's 29th Annual Meeting in Dallas in June 2001 and were summarized in the Book and Paper Group Annual 20. REFERENCESAlbertsson, U., and O.Samuelson. 1962. A colorimet-ric method for the determination of carbonyl groups of cellulose. Analytica Chimica Acta27:434–40. ASTM. 1962. Standard test method for intrinsic viscosity of cellulose, D1795-62. Philadelphia: American Society for Testing and Materials. ASTM. 1963. Standard test methods for carboxyl content of cellulose, D1926-63. Philadelphia: American Society for Testing and Materials. Bansa, H.1998. Aqueous deacidification—with calcium or magnesium? Restaurator19:1–40. Bogaard, J., and P. M.Whitmore. 2001. Effects of dilute calcium washing treatments on paper. Journal of the American Institute for Conservation40:105–23. Burgess, H., D.van der Reyden, and K.Keyes, comp. 1989. Bleaching. Paper conservation catalog. American Institute for Conservation Book and Paper Group. Washington, D. C.: AIC. Chap. 19: 12. Davidson, G. F.1948. The acidic properties of cotton cellulose and derived oxycelluloses. Part 2, The absorption of methylene blue. Journal of the Textile Institute39: T65–T86. Head, F. S. H.1955. The reduction of the aldehyde groups in hydrocelluloses by sodium borohydride. Journal of the Textile Institute46:T584–T586. Helfferich, F.1962. Ion exchange. New York: McGraw-Hill. 83–84. Hey, M.1979. The washing and aqueous deacidifica-tion of paper. Paper Conservator4:66–80. Luetzow, A. E., and O.Theander. 1974. 6-aldehydo-celluloses—thermal instability, �-elimination and acid hydrolysis. Svensk Papperstidning77:312–18. Ohlsson, A., and S.Rydin. 1975. Washing of pulps. Part 2, The sorption of Na, Mg, and Ca on kraft pulp. Svensk Papperstidning78:549–53. Scallan, A. M.1990. The pH inside the fibre wall. In Cellulose sources and exploitation, ed. J. F.Kennedy et al. New York: John Wiley and Sons. 211–15. Tang, L. C.1981. Washing and deacidifying in the same operation. In Preservation of paper and textiles of historic and artistic value II, ed. J. C.Williams. Washington, D. C.: American Chemical Society. 63–86. Tang, L. C.1986. Stabilization of paper through sodium borohydride treatment. In Historic textile and paper materials, ed. H. L.Needles and S. H.Zeronian. Washington, D. C.: American Chemical Society. 427–41. TAPPI. 1988. Hydrogen ion concentration (pH) of paper extracts (cold extraction method), T509 om-88. Atlanta: Technical Association of the Pulp and Paper Industry. Varshney, M. C., and P.Luner. 1961. Reactions of sodium borohydride as applied to pulp and paper. TAPPI Journal44:285–89. Whatman Inc. 1983. Whatman quantitative filter papers support sheet. Clifton, N. J.
Whitmore, P. M., and J.Bogaard. 1994. Determination of the cellulose scission route in the hydrolytic and oxidative degradation of paper. Restaurator15:26–45. Whitmore, P. M., and J.Bogaard. 1995. The effect of oxidation on the subsequent oven aging of filter paper. Restaurator16:10–30. SOURCES OF MATERIALSCupriethylenediamine GFS Chemicals Inc. P.O. Box 245 Powell, Ohio 43065 (800) 858-9682 www.gfschemicals.com Lab supplies; Whatman no. 42 filter paper (large sheets) Fisher Scientific Co. 585 Alpha Dr. Pittsburgh, Pa. 15238 (800) 766-7000 www.fishersci.com Other chemicals Aldrich P.O. Box 2060 Milwaukee, Wis. 53201 (800) 771-6737 www.sigma-aldrich.com Benchtop pH/ISE meter Thermo Orion 500 Cummings Center Beverly, Mass. 01915-6199 (978) 232-6000 www.thermo.com Spectrophotometer (ColorEye Model 7000) Macbeth Division Kollmorgen Instruments Corp. 405 Little Britain Rd. New Windsor, N.Y. 12553 Temperature and humidity chamber Blue M Electric 2218 W. 138th St. Blue Island, Ill. 60406 (708) 385-9000 UV-A fluorescent lamps (UVA-351) Q-Panel Corp. 26200 First St. Cleveland, Ohio 44145 AUTHOR INFORMATIONJOHN BOGAARD has a BS in chemistry from Carnegie Mellon University. He had worked at the Art Conservation Research Center (formerly the Research Center on the Materials of the Artist and Conservator) since 1978, where his primary area of research was paper chemistry. Address: Department of Horticulture, Penn State University, University Park, Pa. 16802 HANNAH R. MORRIS has a PhD in analytical chemistry from the University of Pittsburgh, where her research focused on materials characterization in complex polymer blends using spectroscopy and chemical imaging techniques. Since 2000 she has been deputy director of the Art Conservation Research Center (formerly the Research Center on the Materials of the Artist and Conservator). Address: Art Conservation Research Center, Carnegie Mellon University, 700 Technology Drive, Pittsburgh, Pa. 15219 PAUL M. WHITMORE has a PhD in physical chemistry from the University of California at Berkeley. Following an appointment at the Environmental Quality Laboratory at Caltech studying the effects of photochemical smog on works of art, he joined the staff at the Harvard University Art Museums. Since 1988 he has been director of the Art Conservation Research Center (formerly the Research Center on the Materials of the Artist and Conservator) at Carnegie Mellon University, where his research has been directed toward the study of the permanence of modern art and library materials. Address: As for Morris
 Section Index Section Index |