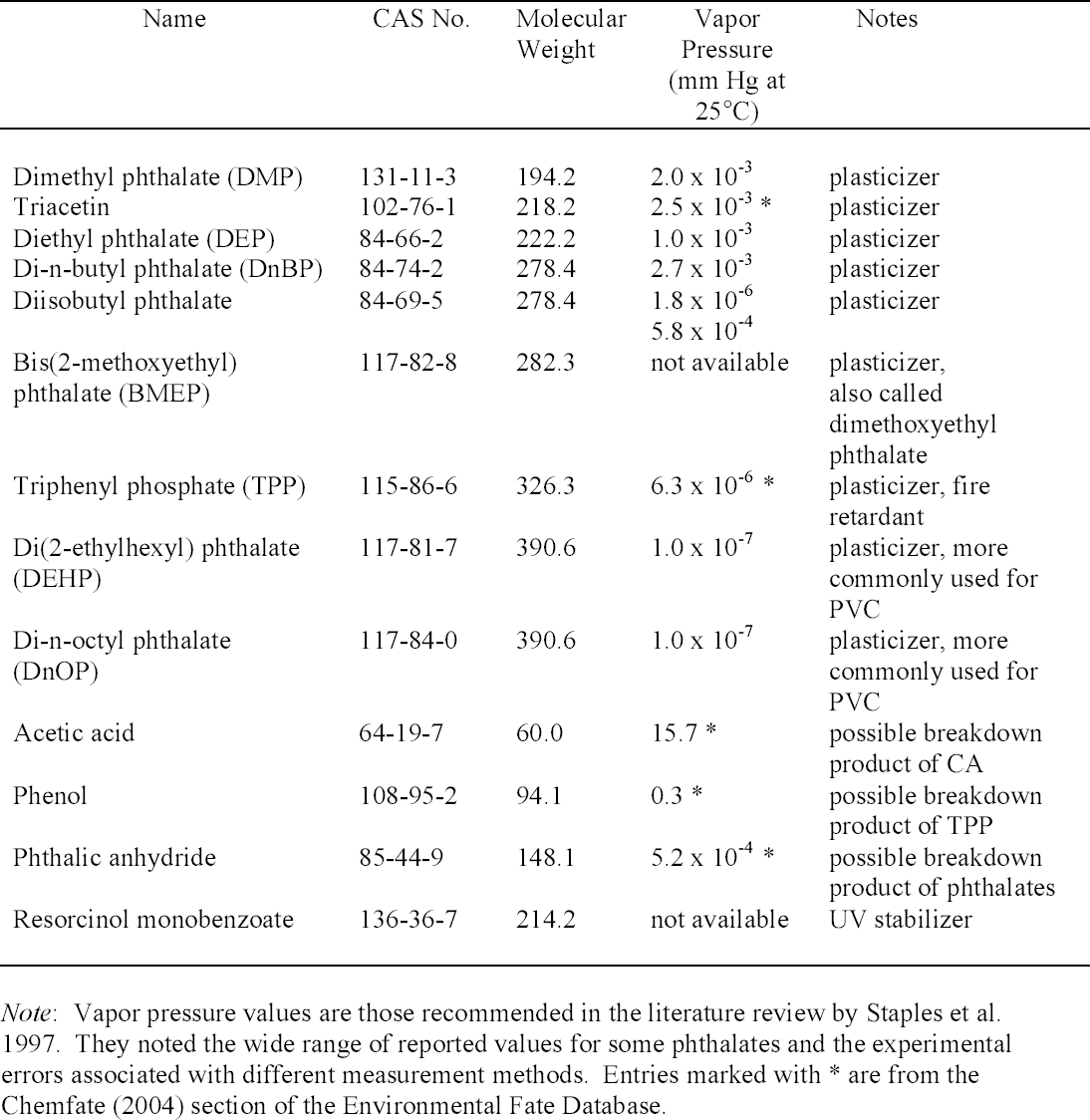
ANALYSIS OF LAMINATED DOCUMENTS USING SOLID-PHASE MICROEXTRACTIONMark Ormsby
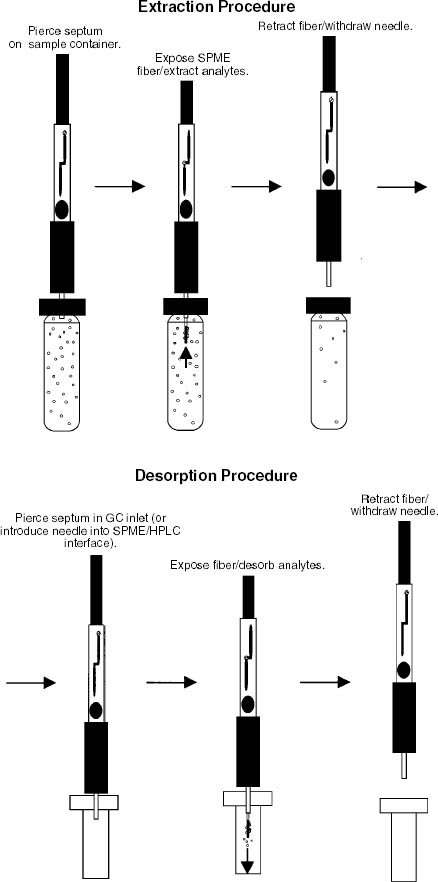
ABSTRACT—To evaluate the condition of a laminated document, it is helpful to identify the plasticizers present in the cellulose diacetate lamination film. Plasticizers degrade more readily than the polymer, and some are less stable than others. Solid-phase microextraction in combination with gas chromatography–mass spectroscopy analysis is a simple, nondestructive, and sensitive technique for studying plasticizers and other additives in laminated documents. In this project, five types of solid-phase microextraction fibers were used to study films that contained various combinations of plasticizers. Laminated documents related to the Louisiana Purchase were also studied. Plasticizers were easily identified, as well as the possible degradation products phthalic anhydride and phenol. Various types of films were readily distinguished. With further study it may be possible to better understand the deterioration process by relating the original composition of the cellulose diacetate film to the present condition of a laminated document. TITRE—L'analyse de documents lamin�s par la microextraction en phase solide. R�SUM�—Afin d'�valuer l'�tat d'un document lamin�, il est utile d'identifier les plastifiants pr�sents dans les films de laminage en diac�tate de cellulose. Les plastifiants d�gradent plus facilement que le polym�re, et certains sont moins stables que d'autres. La microextraction en phase solide combin�e avec l'analyse par spectrom�trie de masse/chromatographie en phase gazeuse est une technique simple, non destructive et tr�s sensible pour �tudier les plastifiants et autres additifs dans les documents lamin�s. Dans ce projet, cinq diff�rentes fibres pour la microextraction en phase solide ont �t� utilis�es pour �tudier des films qui contenaient diverses combinaisons des plastifiants. Des documents lamin�s traitant de l'achat de la Louisiane ont �t� �galement �tudi�s. Les plastifiants ont �t� facilement identifi�s, ainsi que de l'anhydride phtalique et du ph�nol, qui sont vraisemblablement des produits de d�gradation. Il est ainsi facile de distinguer les divers types de films de laminage utilis�s. Avec davantage de recherches, il pourra �tre possible de mieux comprendre le processus de d�t�rioration en �tablissant la corr�lation entre la composition initiale des films de diac�tate de cellulose et l'�tat actuel du document lamin�. T�TULO—An�lise de documentos laminados utilizando microextra��o em fase s�lida. RESUMO—Para avaliar a condi��o de um documento laminado, � �til identificar os plastificantes presentes no filme de diacetato de celulose utilizado para laminar. Os plastificantes degradam-se mais rapidamente do que o pol�mero, e alguns s�o menos est�veis que outros. A microextra��o em fase s�lida combinada com an�lise de espectroscopia cromatogr�fica gasosa � uma t�cninca simples, n�o-destrutiva, e sens�vel para o estudo de plastificantes e outros aditivos utilizados na lamina��o de documentos. Neste projecto a microextrac��o em fase s�lida foi utilizada em cinco tipos de filmes que continham v�rias combina��es de plastificantes. Documentos laminados relacionados com com o Rendimento da Louisiana tamb�m foram estudados. Os plastificantes foram facilmente identificados bem como a poss�vel degrada��o de produtos como o anidrido ft�lico e o fenol. V�rios tipos de filmes foram rapidamente distinguidos. Atrav�s de estudos adicionais talvez seja poss�vel entender melhor o processo de deteriora��o, em se relacionando a composi��o original do filme de diacetato de celulose com a condi��o atual de um documento laminado. TITULO—An�lisis de documentos laminados utilizando microextracci�n de fase solida. RESUMEN—Para evaluar la condici�n de un documento laminado, es �til identificar los agentes plastificantes presentes en la pel�cula de diacetato de celulosa con la que fueron laminados. Los plastificantes se degradan mas r�pidamente que el pol�mero y algunos son menos estables que otros. La t�cnica de microextracci�n en fase solida combinada con la espectrometr�a de masas-cromatograf�a de gases, es una tecnica simple, no destructiva y sensible para estudiar los plastificantes y otros aditivos presentes en los documentos laminados. En este proyecto se utilizaron cinco tipos de fibras para microextracci�n en fase solida para estudiar las pel�culas quen conten�an varias combinaciones de plastificantes. Los documentos laminados referentes a 1 INTRODUCTIONThe National Archives and Records Administration (NARA) was one of the first institutions to employ cellulose diacetate (CA) lamination on a large scale as a means of preserving documents (Stiber 1988). A heated hydraulic press was used to bond a thin film of CA to the document. This treatment was used from the 1930s to the 1980s, and during this time the laminating procedure evolved, including changes in the use of tissue reinforcement and deacidification. The formulation of the CA lamination film also varied. At least three different brands of films were used (Gear 1965), and the composition of these may have changed over time, depending on the manufacturer's formulation and quality control (Barrow 1965; Clements 1972). The main difference between these films and others available was in the type and amount of plasticizers added to lower the softening temperature of CA (Gear 1957). Without these additives, the heat and pressure needed to melt the film for lamination would damage the paper (Wilson and Parks 1983). CA lamination film typically contains 20–30% plasticizers by weight (Stannett 1950). Table 1 lists phthalates and other additives commonly used with CA lamination film and some possible breakdown products. The National Bureau of Standards (NBS, now the National Institute of Standards and Technology) conducted several research projects focusing on document lamination beginning in 1933 and culminating in 1959 with specifications for archival lamination films (Wilson and Forshee 1955, 1959a, 1959b). Research by NBS and others showed that some plasticizers are more stable than others. In fact, the plasticizer is typically more susceptible to degradation than the CA polymer (DeCroes and Tamblyn 1952; Shinagawa et al. 1992). Therefore, to evaluate the condition of a laminated document, it is helpful to identify the plasticizers present and any breakdown products. This information may provide insight into the long-term stability of the item. For example, documents may be delaminated to repair damage, to remove degrading CA, or to prepare them for exhibit. An acetone bath is typically used to remove the CA, but occasionally more polar solvents are necessary to delaminate some documents (Page 2003). This change in solubility indicates that the CA has lost some degree of acetylation, which in turn may affect the compatibility of a plasticizer with the polymer (Stannett 1950). This deterioration could be due to a number of factors, including the composition of the film, the procedures used for lamination, and the subsequent exhibit and storage conditions. By studying the relationship between the composition of the CA and the present condition of the documents, it may be possible to better understand the deterioration process. This knowledge may help to more fully evaluate the current condition of documents and to anticipate potential problems with collections laminated with various types of film. For NARA, identifying the plasticizers can also suggest when a document was laminated, because the time periods during which different films were used are known. Since the lamination procedure evolved over time, knowing the treatment date may provide additional information about whether the document was deacidified before lamination and what other procedure may have been performed. One commonly used method for identifying plasticizers is Fourier transform infrared (FTIR) spectroscopy (Haslam and Willis 1965; Ballany et al. 1998). Because CA has strong peaks in its FTIR spectrum that can overlap and obscure peaks from the plasticizers, it is often necessary to extract the plasticizers from the document. In addition, many of the commonly used plasticizers have similar spectra, so it can be difficult to positively identify a specific compound. The sample may also contain a mixture of plasticizers and other additives, further complicating the spectral interpretation. Solid-phase microextraction (SPME) in combination with analysis by gas chromatography–mass spectrometry (GC-MS) is a convenient alternative method for identifying plasticizers. Invented in 1989 by Janus Pawliszyn and coworkers, SPME is a simple, sensitive, and economical technique that has become popular in a This article describes the use of SPME–GC-MS for identifying plasticizers and their breakdown products in CA films and laminated documents. Various sampling methods were explored to refine the procedure. Laboratory samples prepared by the NBS as part of its earlier research were studied, as well as other materials in the collection of the NARA Research and Testing Laboratory. These techniques were applied to documents related to the Louisiana Purchase that had been laminated in the 1930s. These documents showed signs of CA degradation because they could be delaminated only by adding water to the acetone bath. 2 BACKGROUND2.1 SOLID-PHASE MICROEXTRACTIONOne of the principal advantages of SPME is its simplicity (Vas and Vekey 2004). In one solvent-free step, the SPME fiber collects and concentrates the sample, and the fiber is then used to transfer the sample to the instrument for analysis. Other sampling procedures may require several time-consuming steps using solvents and expensive equipment while increasing the potential for sample loss and contamination. In many cases, the detection limits with SPME are also lower than with other methods. The procedure for collecting the sample is shown in the top row of figure 1 (Supelco 1999). Similar to a syringe, a SPME fiber has a 1 cm length of fused silica attached to a stainless steel plunger. The tip is coated with a polymer and is shielded inside a hollow needle. When the plunger is depressed, the fiber extends, the polymer is exposed, and the sample is collected onto it by absorption or adsorption, depending on the type of coating. After a suitable exposure time (a few seconds to a few hours or even weeks, depending on the application), the fiber is retracted. The bottom row of figure 1 illustrates the process for transferring the sample for analysis. The fiber is inserted into the GC-MS, and the plunger is depressed to expose the polymer. The heated injector port drives off the collected compounds, which then flow into the instrument for qualitative or quantitative analysis. Since the fiber has been “cleaned” by heating in the injector, it is ready to be reused.
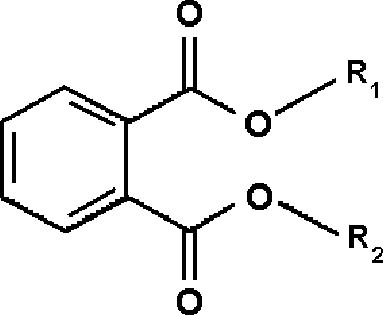
With direct immersion (DI-SPME) sampling, the fiber is inserted into a liquid. In headspace (HSSPME) sampling, the fiber collects compounds from the headspace above the sample. HS-SPME reduces interferences from the sample matrix and is often used for analysis of soils, wastewater, blood, and other complicated matrices (Vas and Vekey 2004). The amount of sample collected on the SPME fiber depends on a number of factors (Supelco 2001). As with GC columns, a fiber coated with a polar polymer is more sensitive to polar compounds, and 2.2 ADDITIVES IN LAMINATION FILMSPlasticizers are compounds that dissolve in and modify the properties of a polymer but are not chemically bound to it (Williams 1993). Low molecular weight phthalates (fig. 2) are commonly used to plasticize CA (Stannett 1950) as well as other polymers. They have become widespread environmental contaminants because they readily leach or volatilize from plastic products during use or after disposal (Staples et al. 1997). Table 1 lists phthalates and other additives commonly found in
In addition to plasticizers, the CA film may contain a number of other additives. The NBS specification (Wilson and Forshee 1959b) for archival laminating film recommended a minimum of 0.5% by weight of an acid acceptor such as magnesium acetate, as well as an antioxidant. A minimum of 1% by weight of an ultraviolet absorber was also recommended. To effectively use SPME to study the variety of plasticizers and other additives in lamination films, it is necessary to determine which fibers are most sensitive for these compounds. A set of CA films was studied systematically by both HS-SPME and DISPME. These samples were prepared by NBS as part of their research programs in the 1950s and are now in the files of the NARA Research and Testing Laboratory. These results were applied to SPME analysis of actual laminated documents.
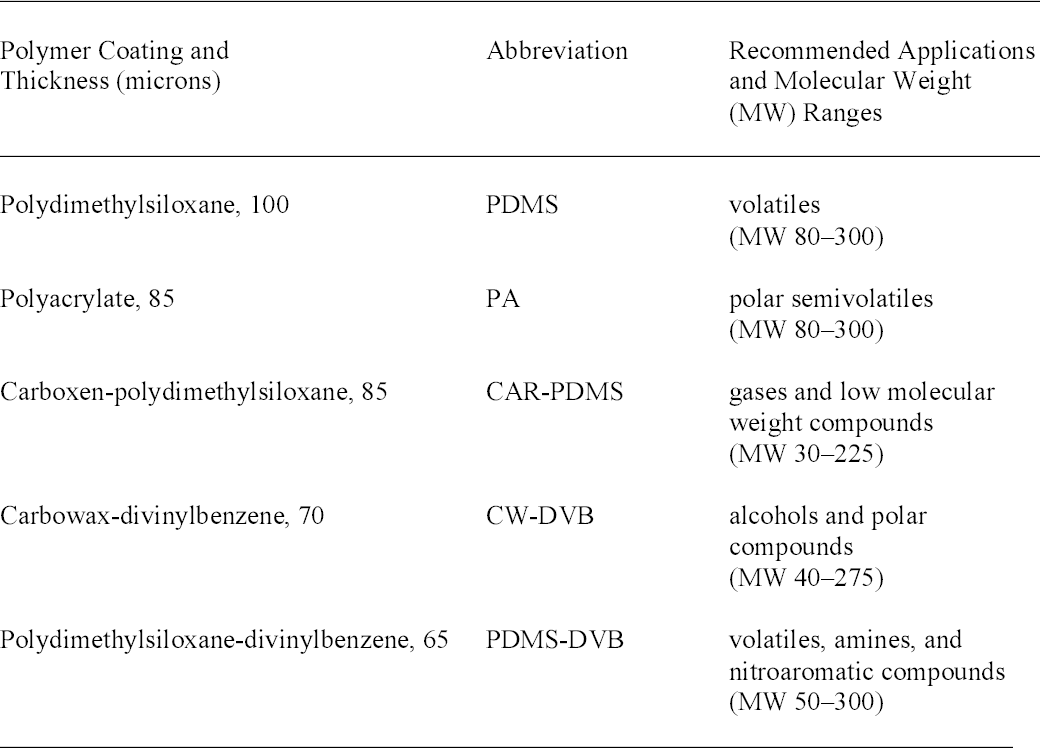
3 PROCEDUREFive types of SPME fibers (from Supelco) with different polymer coatings were evaluated. Table 2 lists the fibers and their recommended applications. The fibers were initially conditioned by heating in the GC-MS injector at a temperature as recommended by the manufacturer. Analysis was performed on an HP6890N/5973 Inert GC-MS with a 30-meter HP5-MS (5%phenyl)-methylpolysiloxane column (Agilent). A Supelco narrow-bore inlet liner optimized for
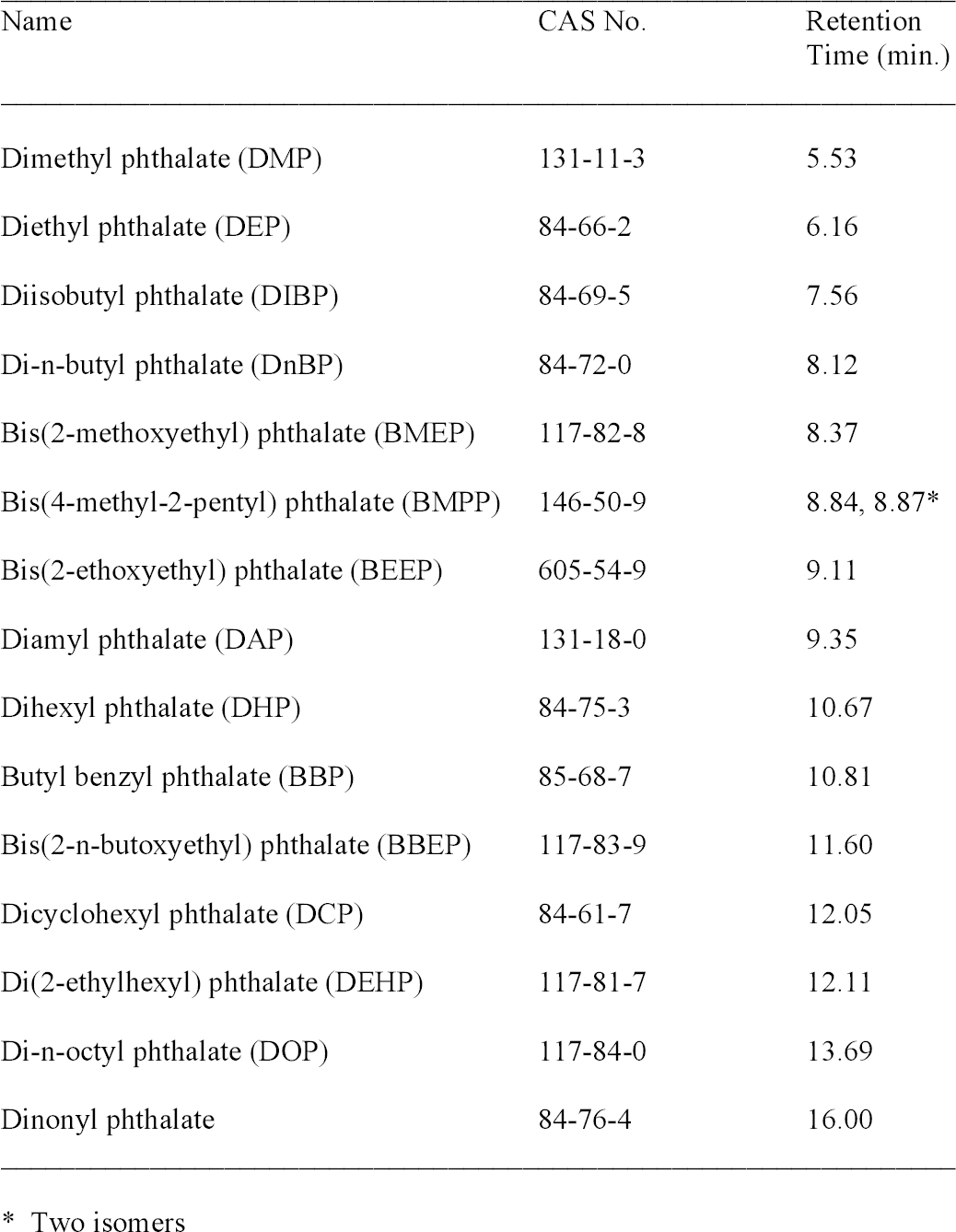
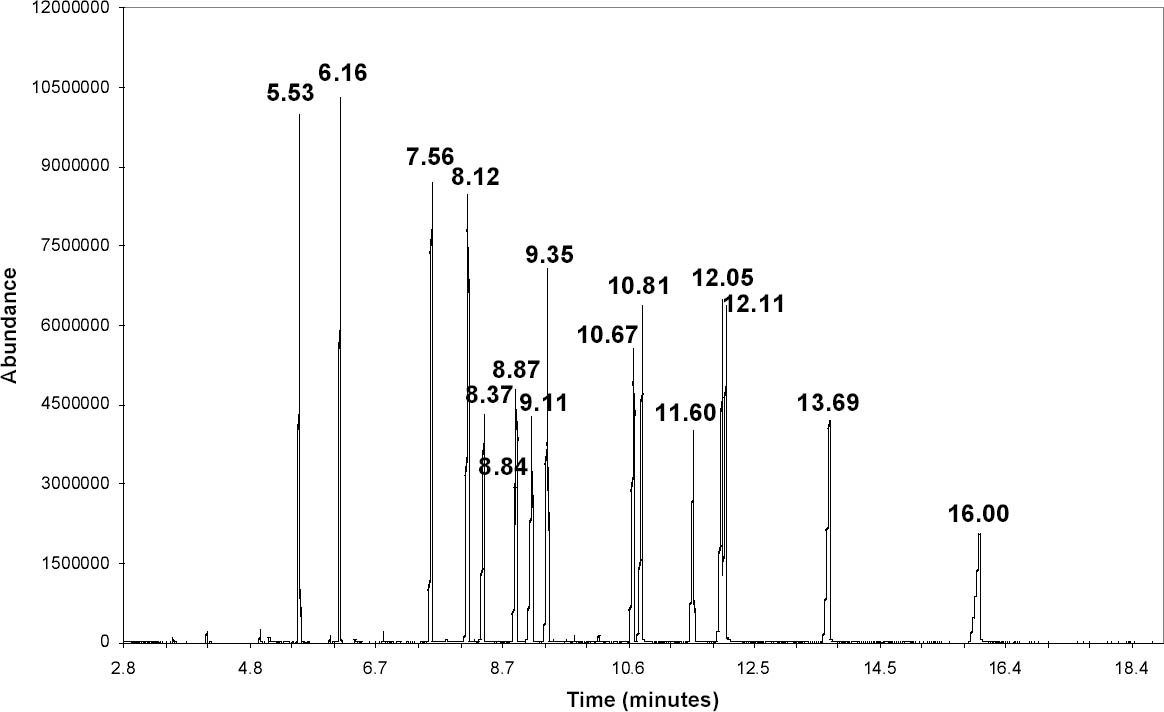
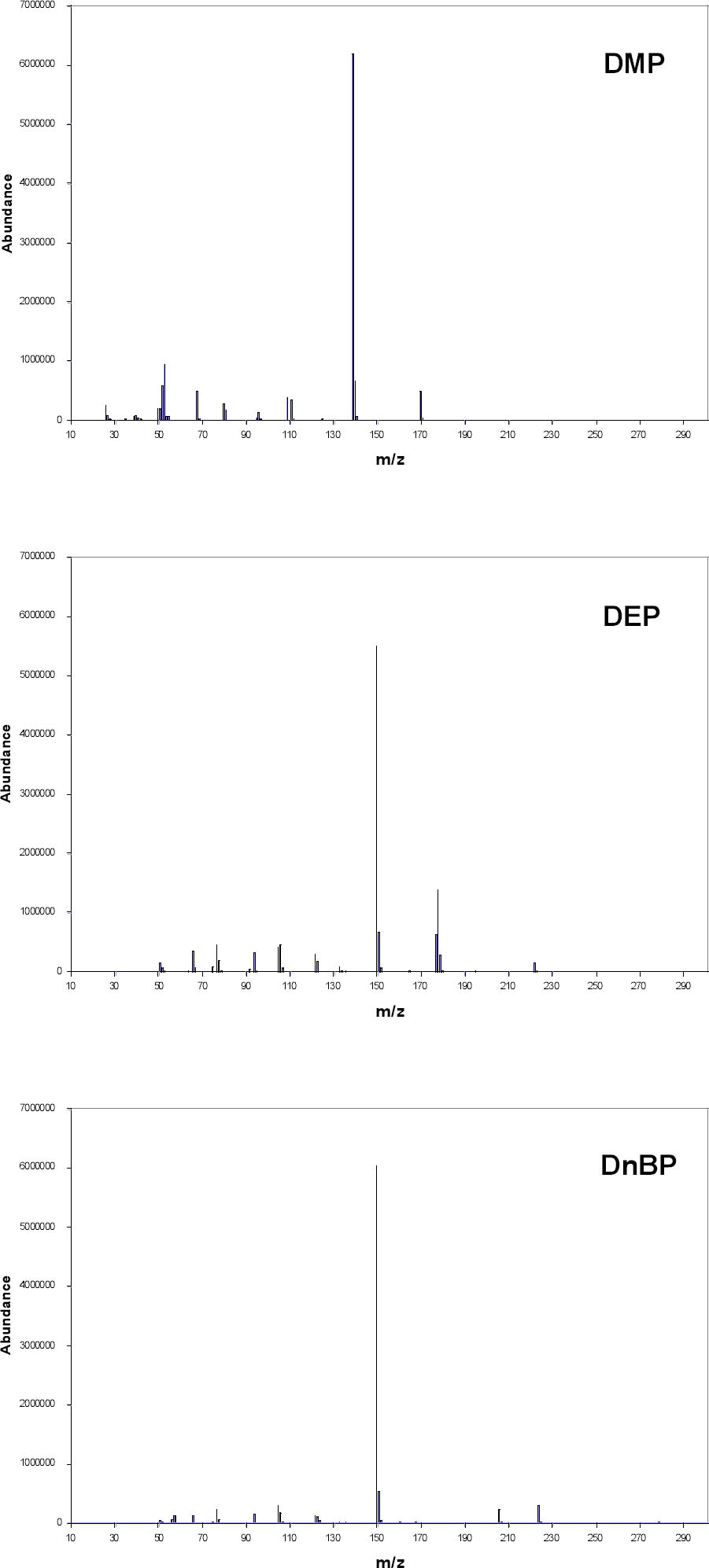
A splitless injection was made with the injector port at 250�C. The carrier gas was helium at 1 ml/minute in the constant flow mode. The oven temperature was initially 50�C. It was heated at 30�C/minute to 210�C and then raised at 10�C/minute to 275�C, where it was held for seven minutes. Mass spectra were collected using electron ionization and a quadrupole mass filter. The MS source, quadrupole, and detector temperatures were 230�, 150�, and 280�C, respectively. A standard spectra autotune using perfluorotributylamine was performed for comparison with the National Institute of Standards and Technology/Environmental Protection Agency/National Institutes of Health (NIST/EPA/NIH) Mass Spectral Library 2002 (Agilent). Spectra were collected in the scan mode over the range 35–400 amu. A standard solution (NSI Solutions) containing 15 phthalates at approximately 1 mcg/ml concentration in hexane was used to optimize the GC-MS settings. To reduce the baseline from the hexane, the lower end of the mass range was raised to 45 (compared to 35 for all other data). Samples were collected using DI-SPME. The total ion chromatogram (TIC) in figure 3 shows that these 15 compounds can be distinguished based on the retention time, the time required for a peak to elute from the column. Retention times were reproducible to approximately � 0.03 minute. An unknown can possibly be identified by determining its retention time (table 3). This set of standards does not include all possible phthalates, however, and it is possible that a different compound might have the same retention time as one of the standards. The identity of a peak can possibly be clarified by examining its electron ionization mass spectrum (fig. 4) and comparing it to the spectral library. Ideally, the mass spectrum serves as a fingerprint for a specific compound, but this is not true in all cases. Di-n-butyl phthalate (DnBP) and some other phthalates have mass spectra with few features besides a large peak at mass-to-charge ratio (m/z) 149. This peak is common to all alkyl phthalates with alkyl
As part of its research projects in the 1950s, NBS prepared experimental CA films made with various combinations of plasticizers and other additives. Samples of these films were taken by cutting a small piece approximately 1 cm2 and placing it in a 10 ml glass vial capped with a polytetrafluoroethylene/silicone septum (Supelco). A SPME fiber was inserted through the septum, exposed to the headspace for 10 minutes, removed, and immediately analyzed. Preliminary testing indicated that the 10-minute exposure provided an adequate signal in a relatively short time. The sensitivity of this method could be improved by using a stir bar or heat (Supelco 1999). This procedure was repeated for five SPME fiber types and various experimental films. To check for carryover of sample on a fiber, a blank run was made between each exposure to a film. To compare the sensitivity of the fibers, the area of the plasticizer peaks was calculated using the ChemStation analysis program. These qualitative tests indicated that the polyacrylate (PA), carbowax-divinylbenzene (CWDVB), and polydimethylsiloxane-divinylbenzene (PDMS-DVB) fibers were suitable for HS-SPME detection of the plasticizers dimethyl phthalate (DMP), diethyl phthalate (DEP), and triacetin as well as phenol, discussed below. These results agree with a study of fiber types used for detecting phthalates in water (Penalver et al. 2000). The PDMS fiber was much less sensitive to phenol. For all four compounds the carboxen-polydimethylsiloxane (CAR-PDMS) fiber had much lower sensitivity. It may be useful, however, for studying the acetic acid
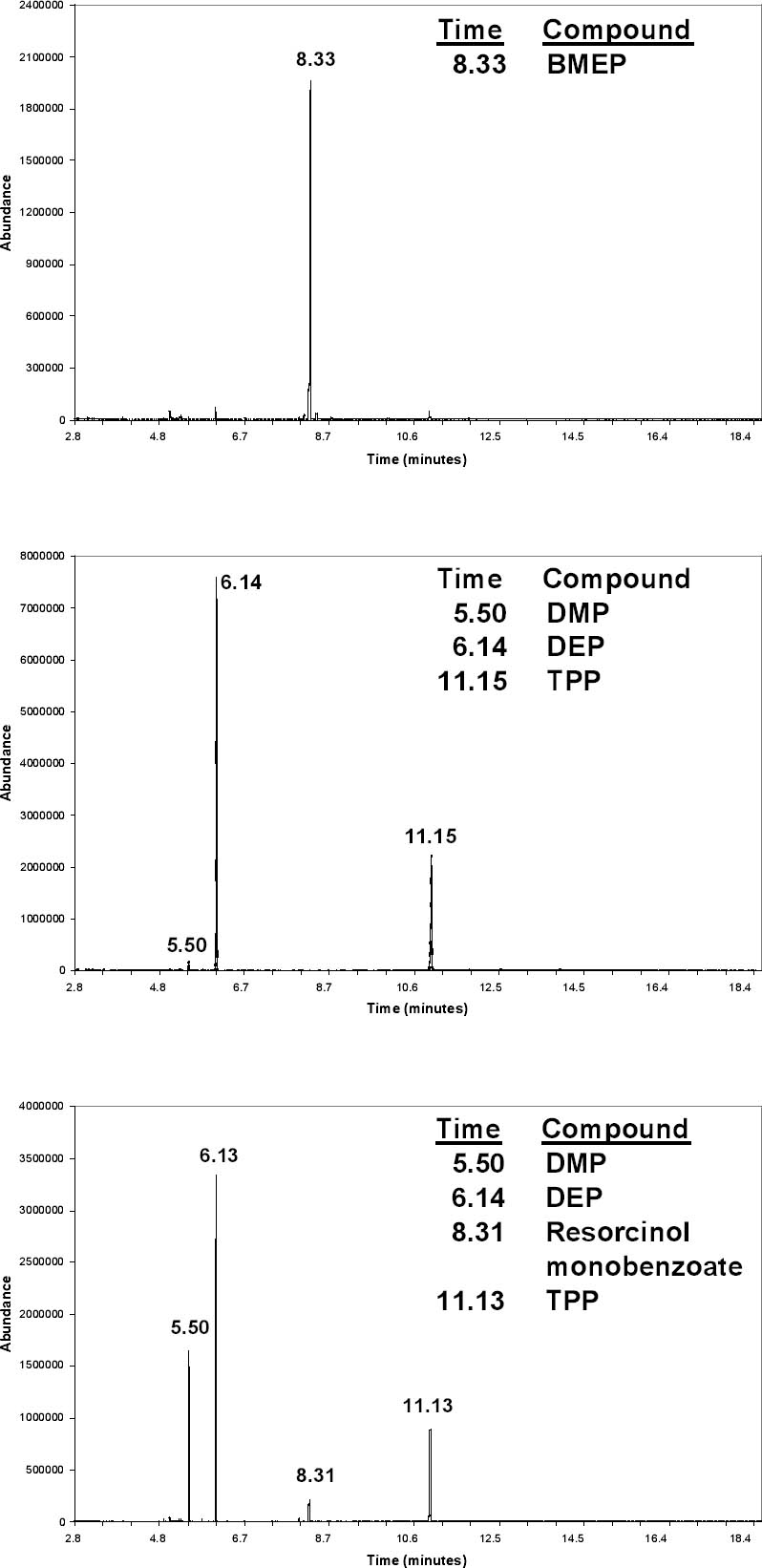
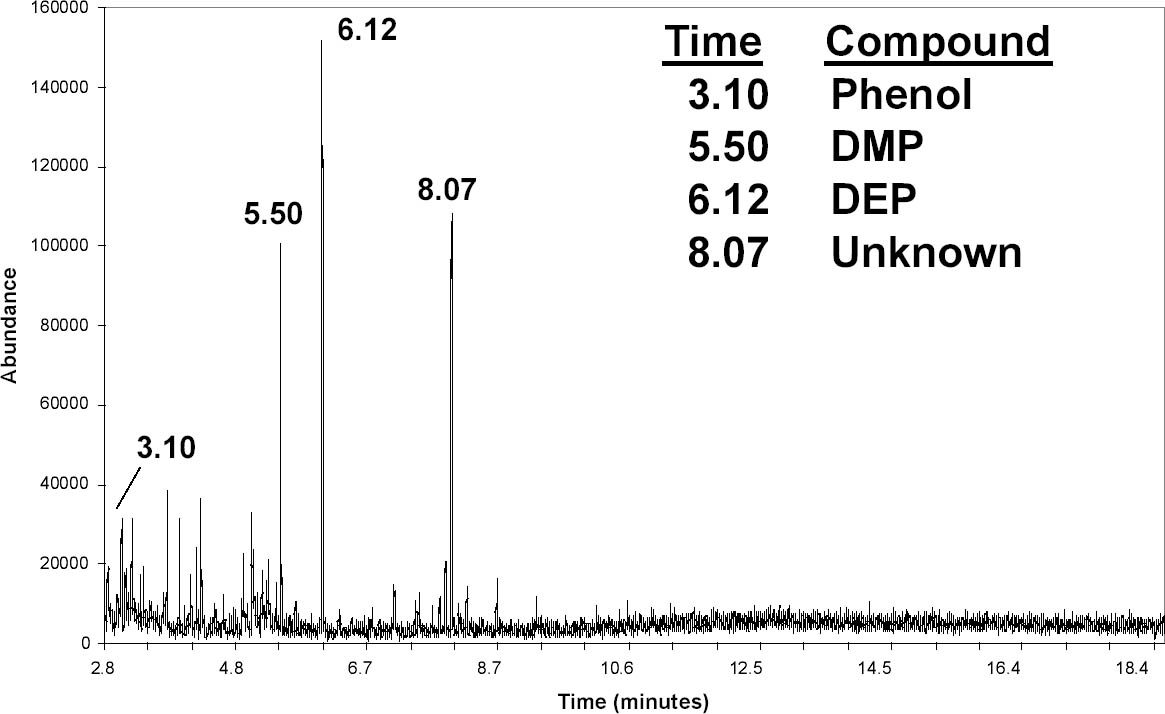
The procedure was modified for documents and objects from which a sample could not be cut and placed in a vial. For HS-SPME, a small beaker or petri dish top was placed on the document, and the fiber was exposed by slipping it under the edge of the glass (fig. 5). When possible, a small drop of ethanol was placed on the document for DI-SPME. TPP was easily detected using this method, although it usually was not found with HS-SPME because of its low volatility. This procedure was assumed not to cause damage to the document because of the minimal exposure to ethanol, but, as a precaution, the drop was placed on the border of laminated documents in an area that contained only the plastic. 4 RESULTS AND DISCUSSIONFigure 6 shows the TICs from three lamination films that were sampled by DI-SPME with a PDMS-DVB fiber and an ethanol drop. The upper graph is from a sample of DuPont 88CA48 film that NARA used from the 1940s to 1956. The largest peak was identified as bis(2-methoxyethyl) phthalate (BMEP) based on its retention time and comparison with the spectral library. (With the 1992 edition of the NIST/EPA/NIH database, BMEP and DnBP could not be distinguished. The 2002 version has an additional peak at m/z 59 that dominates the BMEP spectrum.) NBS research showed that BMEP is vulnerable to degradation (Wilson and Forshee 1959a). As a result of these studies, NARA switched to P911 film from Celanese Corporation of America in 1956 and continued using this film into the 1980s (Gear 1965). The middle graph in figure 6 shows a TIC from P911 film. The three largest peaks were identified from their mass spectra as DMP, DEP, and TPP. The bottom graph shows XCA 14672, another film manufactured by Celanese Corporation. It has an additional peak from resorcinol monobenzoate, an ultraviolet absorber recommended in the NBS specifications (Wilson and Forshee 1959b). Thus, figure 6 illustrates that with DI-SPME these three films can be easily and quickly distinguished. Important data about their composition that might be relevant in evaluating their condition and susceptibility to degradation are also gathered. As discussed in the introduction, it might be possible to gather this information by FTIR analysis of various solvent extractions (Haslam and Willis 1965), but such procedures are likely to be more difficult and time consuming. Figure 7 shows the results of HS-SPME from a newspaper laminated in the first run of NARA's presses in 1936. A PDMS-DVB fiber was exposed for 40 minutes using the setup shown in figure 5. The background is noticeably higher compared to the DISPME data, and peaks from the glass beaker are also present. As noted above, the signal-to-noise ratio could be improved by increasing the agitation in the headspace. Along with DMP and DEP, the peak at 8.07 minutes might be a phthalate based on the m/z
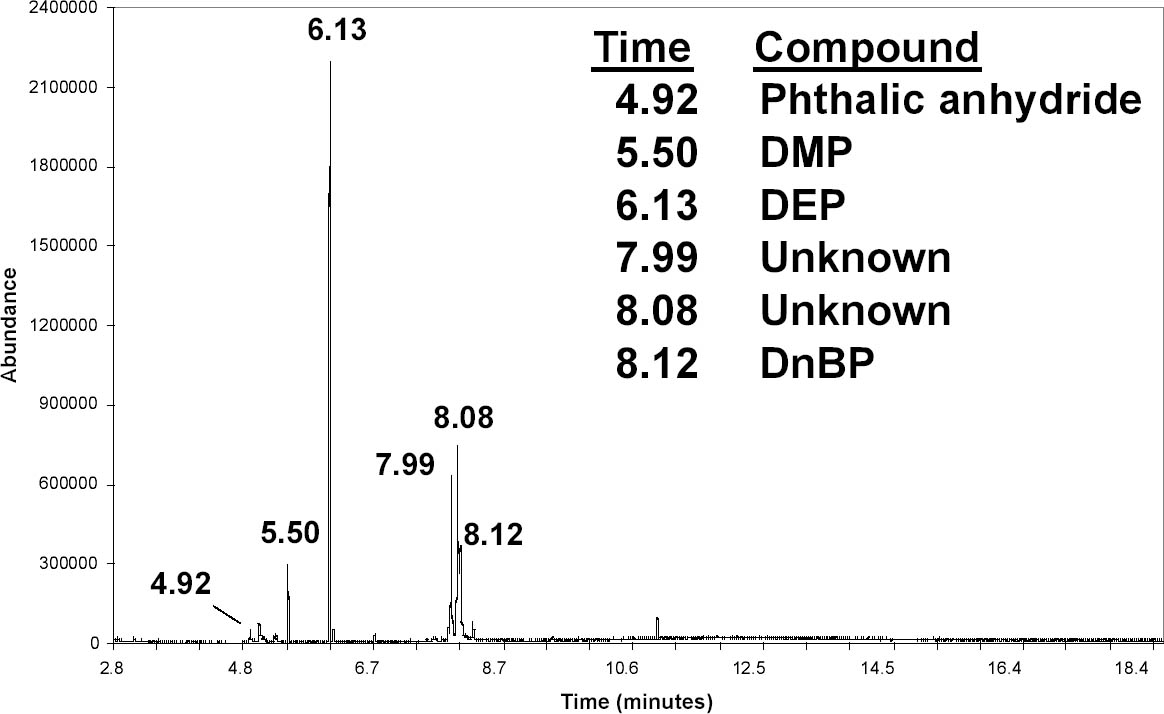
A group of documents related to the Louisiana Purchase had been treated and returned to storage at least one year before being analyzed with SPME. As noted in the introduction, it was known that the CA had deteriorated to some degree because water had to be added to the acetone bath in order to delaminate these documents. Scraps of CA had been trimmed from the edges of the film before bathing them. These scraps had been saved and were sampled using a drop of ethanol. As shown in figure 8, DMP, DEP, and DnBP were found. As with the newspaper, there were two other peaks that could not be positively identified. They both had strong signals at m/z 149, which suggests that they are phthalates. A small peak from phthalic anhydride was also found in several CA scraps. It is used to synthesize many phthalates (Wilson 1995), and its presence may indicate that a plasticizer is degrading (Shashoua 2001). In this project phthalic anhydride has been detected only in early CA films from the 1930s or in films that exhibited signs of deterioration. The brand of CA film used on these Louisiana Purchase documents has not yet been identified, but, as can be seen by comparison with figure 6, it is not the 88CA48 film that NARA began using in the 1940s. The degree to which the composition of this early laminating film is related to its deterioration is unclear. This question might be clarified by analyzing other documents laminated at different times and evaluating the solubility of those films. Quantitative measurements of phthalic anhydride hydride, phenol, and other degradation products might also be correlated with the condition of the documents. If successful, it might be possible to use SPME to quickly test a set of laminated documents, evaluate their condition, and identify those most susceptible to deterioration. Such further study will aid in understanding the complex process of CA degradation. Although much accelerated aging research has been published on this topic, it is still difficult to evaluate the role of specific plasticizers. For example, although NBS research showed that BMEP is unstable, an analysis of a laminated document did not reveal the expected breakdown products (Conley 1998). Also, although TPP acts as a stabilizer in cellulose diacetate lamination films (Wilson and Forshee 1959a), it has been shown to play an important role in the degradation of cellulose triacetate photographic film (Shinagawa et al. 1992). Because of differences in materials, aging conditions, and analysis techniques, it is difficult to compare the results of different studies. In addition, a particular plasticizer may have different effects on the initial and secondary degradation reactions (Wilson and Forshee 1959a). Since most lamination films contain mixtures of plasticizers and other additives, the issue is complicated further. To supplement the accelerated aging research, it might be helpful to study some of the vast number of laminated documents that have been stored for decades at ambient conditions. SPME could be used to efficiently analyze the composition and degradation products of a range of documents laminated at different times with different films. Finally, one of the Louisiana Purchase documents was sampled more than one year after it had been delaminated. The data, not shown, were taken on an older GC-MS system using the same type of column but different instrument settings. A PA fiber was placed on the document, covered with the top of a petri dish, and exposed for 75 minutes. DEP was detected as well as other compounds. Thus, analysis by HS-SPME may provide information about previous treatments. Besides lamination, this testing might reveal previous application of volatile pesticides (Ferrari et al. 2004). 5 CONCLUSIONSThis project demonstrates that SPME–GC-MS is a very useful technique for studying plasticizers and other additives in CA laminated documents. The method is simple and sensitive, and data can be collected with minimal risk to the objects. Several common plasticizers and additives were easily identified using DI-SPME with ethanol. Various brands of CA film could be readily distinguished. HS-SPME also provided useful data with a nondestructive method. Both techniques detected products from the breakdown of plasticizers. With further research it may be possible to use the SPME procedure to better understand the relationship between the original composition of the lamination
These techniques for studying plasticizers in laminated documents can easily be adapted to many other types of objects and compounds. Thus far, the majority of work with SPME in conservation has focused on gaseous pollutants, but there are many other potential applications for this simple yet versatile technique. ACKNOWLEDGEMENTSI would like to thank my colleagues in the Research and Testing Laboratory and the Document Conservation Laboratory at National Archives and Records Administration for their assistance and suggestions. In particular, I would like to thank Susan Page, who treated the Louisiana Purchase documents and suggested the SPME analysis. My thanks also to the late William K. Wilson, National Bureau of Standards chemist, for sharing his vast knowledge of lamination and preservation. REFERENCESBallany, J., D.Littlejohn, R. A.Pethrick, and A.Quye. 1998. Probing the factors that control degradation in museum collections of cellulose acetate artefacts. In Historic textiles, papers, and polymers in museums, ed. J. M.Cardamone and M.Baker. American Chemical Society Symposium series 779, vol. 12. Washington, D. C.: American Chemical Society. 145–165. Barrow, W. J.1965. Deacidification and lamination of deteriorated documents, 1938–1963. American Archivist28(2): 285–90. Chemfate. 2004. Environmental Fate Database. Syracuse Research Corporation. www.syrres.com/esc/chemfate.htm (accessed June 2004). Clements, R. F.1972. Accelerated aging of cellulose acetate lamination films. Research and Testing Laboratory, National Archives and Records Administration, Washington, D. C. Conley, J.1998. Using LC-MS to preserve a national treasure. Today's Chemist at Work7(2): 43–46. DeCroes, G. C., and J. W.Tamblyn. 1952. Protection of cellulose esters against breakdown by heat and light. Modern Plastics29:27–189. Ferrari, F., A.Sanusi, M.Millet, and M.Monturyl. 2004. Multiresidue method using SPME for the determination of various pesticides with different volatility in confined atmospheres. Analytical and Bioanalytical Chemistry379(3): 476–83. Gear, J. L.1957. Comments on Mr. Turner's article. American Archivist20(4): 329–34. Gear, J. L.1965. Lamination after 30 years: Record and prospect. American Archivist28(2): 293–97. George, C., and H.Prest. 2002. Determination of phthalate esters by positive chemical ionization MS with retention-time locked GC. LCGC North America20(2). www.chromatographyonline.com (accessed September 2003). Glastrup, J.1995. Analysis of gaseous degradation products generated during accelerated aging of cellulose acetate. Research techniques in photographic conservation: Proceedings of the Conference in Copenhagen. Copenhagen: Academy of Fine Arts. 93–95. Haslam, J., and H. A.Willis. 1965. Identification and analysis of plastics. Princeton, N. J.: D. Van Nostrand. Hites, R. A.1997. Gas chromatography mass spectrometry. In Handbook of instrumental techniques for analytical chemistry, ed. F.Settle. Upper Saddle River, N. J.: Prentice Hall. 609–26. Kumar, R.1999. A mass spectral guide for quick identification of phthalate esters. American Laboratory31(November): 32–35.
Lattuati-Derieux, A., S.Bonnassies-Termes, and B.Lavedrine. 2004. Identification of volatile organic compounds emitted by a naturally aged book using solid-phase microextraction/gas chromatography/ mass spectrometry. Journal of Chromatography A1026:9–18. Maines, C.2002. Analysis of exhibition materials. Paper presented at the Eastern Analytical Symposium, Somerset, N. J. Page, S.2003. Cellulose acetate lamination at the National Archives. Part 1: The Louisiana Purchase documents: A case study. In Book and Paper Group Annual22. American Institute for Conservation Book and Paper Group. Washington, D. C.: AIC. 53–59. Pawliszyn, J.1998. Solid phase microextraction: Theory and practice.New York: John Wiley & Sons. Pawliszyn, J. ed.1999. Applications of solid phase microextraction. Cambridge: Royal Society of Chemistry. Penalver, A., E.Pocurull, C.Aguilar, F.Borrull, and R. M.Marce. 2000. Comparison of different fibers in the solid phase microextraction of phthalate esters from water samples. Journal of Chromatography A922(1–2): 377–84. Rhyl-Svendsen, M.2003. Home page of the Indoor Air Pollution Working Group. http://iaq.dk/iap.htm (accessed September 2003). Rhyl-Svendsen, M., and J.Glastrup. 2002. Acetic acid and formic acid concentrations in the museum environment measured by SPME–GC-MS. Atmospheric Environment36:3909–16. Shashoua, Y. R.2001. Inhibiting the deterioration of plasticized poly(vinyl chloride): A museum perspective. PhD diss., Technical University of Denmark, Copenhagen. www.natmus.dk/cons/reports/2001/ys_phd/pvcphd.htm (accessed September 2003). Shinagawa, Y., M.Murayama, and Y.Sakaino. 1992. Investigation of the archival stability of cellulose triacetate film: The effects of additives to CTA support. In Polymers in conservation, ed. N. S.Allen, M.Edge, and C. V.Horie. Cambridge: Royal Society of Chemistry. 138–50. Stannett, V.1950. Cellulose acetate plastics. London: Temple Press. Staples, C. A., D. R.Peterson, T. F.Parkerton, and W. J.Adams. 1997. The environmental fate of phthalate esters: A literature review. Chemosphere35(4): 667–749. Stiber, L.1988. The delamination of the Washington and Lee ledger. Part 1: An overview of cellulose acetate lamination. In Early Advances in Conservation, ed.V.Daniels. British Museum Occasional Papers 6. London: British Museum. 27–40. Supelco. 1999. Solid phase microextraction: Theory and optimization of conditions. Bulletin 923A. Supelco, Bellefonte, Pa. Supelco. 2001. Solid phase microextraction troubleshooting guide. Bulletin 928. Supelco, Bellefonte, Pa. Supelco. 2002. Solid phase microextraction application guide CD, 4th ed. Supelco, Bellefonte, Pa. Vas, G., and K.Vekey. 2004. Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. Journal of Mass Spectrometry39:233–54. Williams, R. S.1993. Composition implications of plastic artifacts: A survey of additives and their effects on the longevity of plastics. In Saving the twentieth century: The conservation of modern materials,ed. D. W.Grattan. Ottawa: Canadian Conservation Institute. 135–53. Wilson, A. S.1995. Plasticizers: Principles and practice. London: Institute of Materials. Wilson, W. K., and B. W.Forshee. 1955. Preservation of documents by lamination. National Bureau of Standards, report 4207 to National Archives and Records Service. Washington, D. C. Wilson, W. K., and B. W.Forshee. 1959a. Degradation of cellulose acetate films. Society for Plastics Engineers Journal15: 146. Wilson, W. K., and B. W.Forshee. 1959b. Preservation of documents by lamination.National Bureau of Standards Monograph. Washington, D. C.: Government Printing Office. Wilson, W. K., and E. J.Parks. 1983. Historical survey of research at the National Bureau of Standards on materials for archival records. Restaurator5(34): 191–241. SOURCES OF MATERIALSSPME fibers, inlet liners, and vialsSupelco 595 North Harrison Rd. Bellefonte, Pa. 16823-0048 (800) 247-6628 www.sigma-aldrich.com/supelco GC-MS and HP5-MS columnAgilent Technologies 2850 Centerville Rd. Wilmington, Del. 19808 (800) 227-9770 www.agilent.com/chem 8061 Phthalates Standard MixtureNSI Solutions, Inc. 7517 Precision Dr., Suite 101 Raleigh, N.C. 27617 (800) 234-7837 AUTHOR INFORMATIONMARK ORMSBY graduated from Grinnell College, Grinnell, Iowa, with a degree in physics and received an MS in physics from the University of Maryland. Since 1990 he has worked at the National Archives and Records Administration, initially focusing on conservation applications of image processing. His current research interests include environmental monitoring, studying the role of gelatin sizing in paper degradation, and developing new applications of SPME–GC-MS. Address: National Archives and Records Administration, 8601 Adelphi Rd., Room 1800, College Park, Md. 20740–6001
 Section Index Section Index |