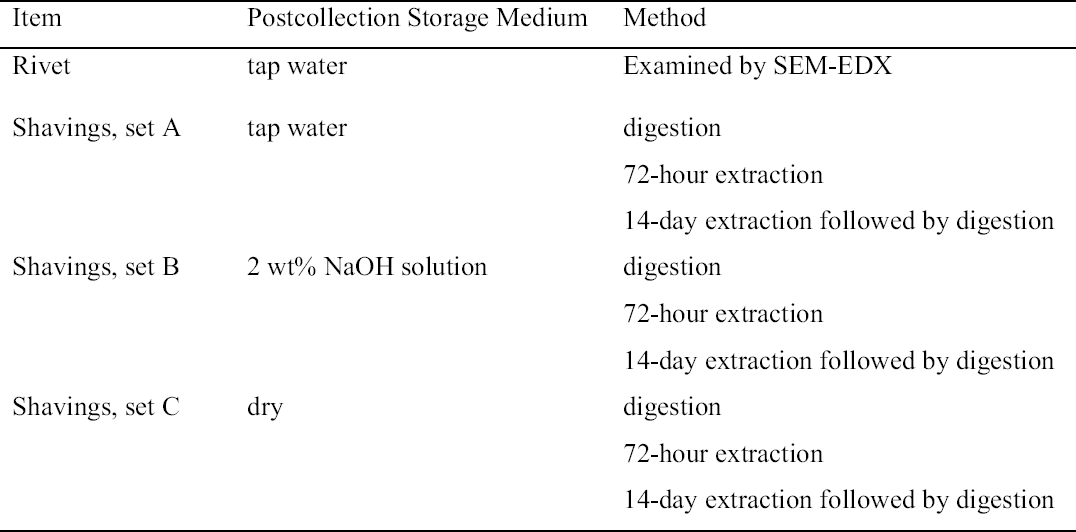
HUNTING FREE AND BOUND CHLORIDE IN THE WROUGHT IRON RIVETS FROM THE AMERICAN CIVIL WAR SUBMARINE H. L. HUNLEYN�STOR G. GONZ�LEZ, PHILIPPE DE VIVI�S, MICHAEL J. DREWS, & PAUL MARDIKIAN
ABSTRACT—The Confederate submarine H. L. Hunley, built in 1863, was successfully recovered in August 2000 after nearly 140 years of immersion in the Atlantic Ocean. One of the most significant challenges faced in the Hunley's stabilization and conservation will be the effective removal of chloride from the corrosion products present on the surface of the submarine. This article addresses the issue of the presence of free and bound chloride in the metal shavings retained when the rivets were removed, by drilling, from the Hunley's hull prior to the excavation of the interior. Before the extraction and digestion studies on the shavings, one of the rivets that had been stored in tap water since its removal was sectioned, and the sectioned surface was characterized using scanning electron microscopy and energy dispersive x-ray analysis. Rivet shavings that were stored dry, in tap water, and in sodium hydroxide made up with tap water after removal were extracted with deionized water, sodium carbonate, and sodium hydroxide solutions for up to two weeks. The total amount of chloride present in each sample was determined using nitric acid digestion. It was found that the shavings stored dry and in tap water contained very similar quantities of total chloride. It was also found that the shavings stored in caustic solution contained significantly less chloride than the other two sets of samples. For all of the shaving samples, it was found that a significant quantity of chloride (> 500 ppm) remained in the corrosion products after extraction, suggesting the presence of some form of bound or occluded chloride ion. TITRE—� la recherche des chlorures libres et li�s dans les rivets en fer forg� du sous-marin H. L. Hunley, navire de la guerre de S�cession am�ricaine. R�SUM�—Le sous-marin conf�d�r� H. L. Hunley, construit en 1863, a �t� renflou� avec succ�s en ao�t 2000, apr�s 140 ann�es d'immersion dans l'oc�an Atlantique. Un des plus grands d�fis du projet de stabilisation et de conservation du Hunley consiste � �liminer les chlorures pr�sents dans les produits de corrosion du sous-marin. Cette �tude s'int�resse � la pr�sence des chlorures, soit libres ou li�s, dans les �chantillons de limailles r�cup�r�s lors de la d�pose par per�age des rivets de la coque du Hunley, avant la fouille de l'int�rieur. Pr�alablement aux �tudes d'extraction sur les �chantillons m�talliques, l'un des rivets, conserv� dans de l'eau de ville depuis qu'il avait �t� r�cup�r�, a �t� sectionn� et analys� � l'aide d'un microscope �lectronique � balayage et microsonde �lectronique. Des solutions d'eau d�ionis�e, de carbonate de sodium et d'hydroxyde de sodium furent utilis�es sur une p�riode de deux semaines pour extraire les chlorures d'�chantillons de limailles de rivets qui avaient �t� stock�s de trois mani�res: au sec; dans de l'eau de ville; et dans une solution d'hydroxyde de sodium. La quantit� totale de chlorures pr�sents dans chaque �chantillon a �t� d�termin�e apr�s digestion dans l'acide nitrique. La quantit� totale de chlorures dans les �chantillons stock�s au sec et dans l'eau douce �tait tr�s similaire. Nous avons �galement montr� que les �chantillons stock�s dans la solution � base de soude contenaient beaucoup moins de chlorures que les deux autres s�ries d'�chantillons. Pour l'ensemble des �chantillons de rivets, une quantit� importante de chlorures (>500 ppm) subsistait dans les produits de corrosion apr�s l'extraction, sugg�rant la pr�sence d'ions chlorures chimiquement li�s. TITULO—La cacer�a de cloruros libres y ligados en los remaches de hierro forjado del submarino H. L. Hunley de la guerra civil de los Estados Unidos. RESUMEN—En agosto del 2000 el submarino Conferado H.L. Hunley, construido en 1863, fue exitosamente recuperado luego de 140 a�os de inmersi�n en el oc�ano Atl�ntico. Uno de los m�s grandes desaf�os en la estabilizaci�n y conservaci�n del Hunley ser� remover cloruros de los productos de corrosi�n presentes en la superficie del submarino. Este art�culo trata la problem�tica de cloruros libres y ligados presentes en limaduras de metal obtenidas cuando remaches del casco del Hunley fueron taladrados y removidos con anterioridad a la excavaci�n de su interior. Previo a los estudios de T�TULO—Cloreto livre e combinado em rebites de ferro forjado do submarino H. L. Hunley da Guerra Civil Americana. RESUMO—O submarino Confederado H. L. Hunley, constru�do em 1863, foi recuperado com sucesso em agosto de 2000 depois de quase 140 anos submerso no Oceano Atl�ntico. Um dos maiores desafios encontrados na estabiliza��o e conserva��o do Hunley foi a remo��o do cloreto dos produtos de corros�o presentes na superf�cie do submarino. Este artigo focaliza a presen�a de cloreto livre e combinado retido nas aparas do metal quando os rebites foram removidos, por perfura��o, do casco do Hunley, antes das escava��es de seu interior. Antes da extra��o e estudos de digest�o das aparas, um dos rebites que tinha sido armazenado em �gua de torneira desde sua remo��o, foi seccionado e a superf�cie seccionada foi analisada usando microscopia de el�tron digital e energia dispersiva do raio-X. As aparas de rebites, que foram armazenadas secas, em �gua de torneira, e em hidr�xido de s�dio produzido com �gua de torneira imediatamente ap�s remo��o, foram extra�das com solu��es de �gua deionizada, carbonato de s�dio e hidr�xido de s�dio por at� duas semanas. A quantidade total de cloreto presente em cada amostra foi determinada usando a digest�o com �cido n�trico. Observou-se que as aparas secas e as armazenadas em �gua de torneira continham quantidades muito similares de cloreto total. Percebeu-se tamb�m que aparas armazenadas em solu��o c�ustica continham quantidades significativamente menores de cloreto do que os outros dois grupos de amostras. Em todas as amostras de rebites encontrou-se que uma quantidade significativa de cloreto (>500ppm) permaneceu nos produtos da corros�o ap�s extra��o, sugerindo a presen�a de alguma forma livre ou fixa do �on cloreto. 1 INTRODUCTIONThe Confederate submarine H. L. Hunley was built in 1863. It sank in February 1864 off the coast of Charleston, South Carolina, shortly after becoming the first submarine in history to sink an enemy ship in combat. The Hunley was successfully recovered essentially intact in August 2000 after nearly 140 years of immersion in the Atlantic Ocean. One of the most significant problems facing the conservator with regard to the conservation of iron artifacts recovered from marine archaeological sites is the removal of the chloride ions present in the corrosion layer and the prevention of subsequent active corrosion. This problem has been the topic of many investigations and remains one of the most active areas in conservation research. While at least 18 different iron corrosion products have been identified on artifacts recovered from burial and marine sites, most of the focus in the literature has been on the chloride (Cl-1) containing corrosion products (Selwyn 1999). The Cl-1 containing compounds most often associated with the corrosion of recovered artifacts include akagan�ite (β-FeOOH), hydrated ferrous chlorides (FeCl2•2H2O and FeCl2•4H2O), and green rust (a mixed-valence ferrous (Fe+2) and ferric (Fe+3) hydroxide and oxyhydroxide of variable Cl-1 content (Gilberg and Seeley 1981; Al-Zahrani 1999; Selwyn 1999). Ferric chloride (FeCl3) has only occasionally been mentioned in the literature and would probably be present only under relatively unusual circumstances Clearly, any successful conservation of an iron artifact must involve the removal of as much Cl-1 as possible. It has been postulated (Turgoose 1982b):
In addition, there has been considerable discussion as to the distribution of the Cl-1 within the corrosion layer. This discussion has recently been reviewed within the context of developing a more sophisticated diffusion model for the removal of Cl-1 during the conservation process (Selwyn et al. 2001). Based on this discussion, it can be argued that the Cl-1 distribution within any artifact can be represented by two boundary cases, one in which it is uniformly distributed throughout the object (North and Pearson 1978b) or one in which a distinct concentration gradient, presumably at the active corrosion layer site, exists (Selwyn et al. 2001). If active corrosion is occurring, it is more reasonable to assume the latter case would represent the Cl-1 within the sample. If the active corrosion has been stopped by treatment, then the former case might better represent the Cl-1 within the artifact. Finally, the effect of treatment conditions on the removal of the Cl-1 from the artifact must be taken into consideration. For this discussion it will be assumed that three general classes of Cl-1 ions may exist within the artifact:
With respect to Cl-1 ions present in the structure of β-FeOOH, in studies employing synthetic akagan�ite it has been postulated that the β-FeOOH is readily transformed under alkaline conditions to α-FeOOH with the release of the trapped Cl-1 ions (North and Pearson 1978b; Al-Zahrani 1999). Other authors have referred to the difficulty of releasing the Cl-1 from the akagan�ite (Turgoose 1982b; Stalh et al. 1998; Selwyn 1999). Perhaps some of this apparent contradiction can be related to the synthetic akagan�ite itself. At least 25 different syntheses producing akagan�ite with chloride contents ranging from 0.87 to 17 wt% have been reported in the literature (Al-Zahrani 1999). In addition, in at least one study on iron Roman nails from a marine site, the ratio of soluble (1) and (2) to insoluble (3) chloride was studied and reported to be approximately 1:4 (Rinuy and Schweizer 1981). Given the critical role that the various Cl-1 containing species are suspected to play in the subsequent corrosion of an artifact, before an appropriate conservation plan can be devised it is important to determine as much as possible about the state of the existing corrosion products. In particular, if possible, the lability of the Cl-1 species present should be characterized. The objective of this investigation was to determine the relative ratios of the class 1 and class 2 to the class 3 Cl-1 species present in the rivets of the H. L. Hunley. For the purposes of this study, any Cl-1 that could readily be extracted from the rivet shavings produced by drilling the rivets during the excavation of the submarine's interior was considered as 2 EXPERIMENTAL2.1 SAMPLE SETSThe samples used in this study were the remnants of the rivets and the metal shavings generated when the rivets were drilled in order to remove them from the hull plates of the H. L. Hunley prior to the excavation of the interior of the submarine. After their removal in February 2001 and until they were employed in this investigation, the rivets from one of the hull plates were stored in tap water and those from the other in 2 wt% sodium hydroxide (NaOH) solutions made up with tap water. In this initial investigation, only rivets stored in tap water were used. The respective rivet shavings were also stored in tap water (set A), and in 2 wt% NaOH (set B) solutions also made up with tap water. When another plate was removed in January 2002, these rivet shavings were allowed to air-dry and stored dry (set C). All the samples and the experiments performed on the three sets of shavings in this investigation are summarized in table 1. The chloride content of the tap water used to store the samples was analyzed periodically. 2.2 RIVET PREPARATION AND ANALYSISTwo rivets that had been stored in tap water were cut into two sections using a Buehler, 5 in. diameter � 0.015 in., diamond wafering blade, cooled with a spray of deionized water, in a Buehler Isomet 2000. After cutting, the rivet halves were mounted on aluminum stubs with carbon tape, and the cut surfaces were examined by scanning electron microscopy (SEM) and energy dispersive x-ray spectroscopy (EDX) using a Hitachi S3500N scanning electron microscope operated at an accelerating voltage of 20 KeV and equipped with an Oxford INCA 400 energy dispersive x-ray analyzer. 2.3 BULK SHAVING SET SAMPLE PREPARATION AND EXTRACTIONFor samples stored in solution, approximately 15 g of the wet shavings were weighed and then dried in an isothermal oven (Fisher Isotemp) at 60oC, cooled, and then reweighed. No quantitative chloride analysis was conducted on the storage solutions because the initial sample volume was not recorded and evaporation during storage was not monitored.
For each individual extraction sample, a carefully selected representative sample of approximately 2.5 g was weighed using a 0.001 g analytical balance (Denver Instruments Model TR-403) into preweighed, 250 ml, high-density polyethylene specimen vials containing 80 ml of either 17 MegOhm deionized (DI) water, or 2.5 wt% sodium carbonate (Na2CO3) (Fisher Certified ACS Reagent Grade, anhydrous) in DI water, or 1 or 2 w% NaOH (Fisher, 50 wt% for Kjeldahl determinations) in DI water. Duplicate samples were extracted for 72 hours or 14 days at room temperature with and without stirring. After extraction, the solid and liquid phases were separated by centrifuging at a relative centrifugal force (RCF) of 1950 (Thermo IEC, Centra Cl-2) for five minutes. The solid phase was then washed with DI water, and the washing solution was added to the supernatant. The washed solid was dried overnight in the isothermal oven at 60�C, cooled to room temperature, and reweighed prior to digestion to determine the Cl-1 remaining after extraction. The supernatant was neutralized with 25 wt% in DI water nitric acid (HNO3) (90 wt%, Fisher Certified ACS Reagent Grade) to a final pH of 6–7.5. The pH was measured using a BDH Gelplas, double junction pH electrode attached to an Orion Model 520 pH meter. After neutralization the solution was concentrated to a final volume of ~ 40 ml on a hot plate at a temperature below the boiling point to avoid the uncontrollable loss of liquid as a result of bubbling. Since all the chloride analysis was done on a w/w basis in terms of parts per million (ppm) Cl-1, the actual final sample volume was not critical, only the final sample mass. 2.4 DIGESTION OF THE SHAVING SET SAMPLESFor each digestion, approximately 0.7 g of dried solid, either before or after extraction, were weighed to �0.001 g into a preweighed 125 ml Erlenmeyer flask, and 25 wt% in DI water HNO3 in a ratio of ~ 20 times the weight of the sample was added. The samples were digested for 48 hours on a hot plate, then cooled to room temperature and neutralized initially with 50 wt% NaOH and then to a final pH of 6–7.5 using 1 wt% NaOH. The supernatant was separated from the solid by centrifugation and then concentrated on a hot plate. The Cl-1 ion concentrations were measured using Hach Quantab, low range, 30–600 ppm, titrator strips as well as by potentiometric titration. For the potentiometric titration, a Thermo Orion 960 autochemistry system equipped with a Thermo Orion Ionplus, double junction Cl-1 ion selective electrode was used. The titrant used was, depending on the Cl-1 concentration, 0.1N, 0.01N, or 0.001N silver nitrate (AgNO3) (made by diluting Fisher Certified 1N standard AgNO3 solution, 1:10, 1:100, 1:1000). The end point was determined using the second derivative method, and the method was calibrated using standard Cl-1 solutions. The limit of detection using this method as it was employed on the samples analyzed in this investigation was experimentally determined to be less than 100 ppm, which corresponds to a value of less than 0.01 wt%. A flowchart of the overall analysis scheme is shown in figure 1. 3 RESULTS AND DISCUSSIONAs part of the process of evaluating the current state of the cast and wrought iron of the hull and its components and prior to beginning the extraction experiments, some preliminary analysis was undertaken on the rivets that were removed as part of the excavation of the interior of the Hunley. For the excavation of the submarine's interior, four hull plates were removed. To remove the hull plates, the rivets holding them in place, approximately 100 per plate, were removed from the outside in. Each rivet was bored and drilled for removal, identified and labeled and then stored in either tap water or a 2 wt% caustic soda solution made from tap water. In addition, the rivet shavings from the boring and drilling were also stored, either dry or in tap water or 2 wt% caustic solution made from tap water. It should be noted that the chloride content of the tap water was monitored over the period covered in this investigation, and it varied from 17 to 30 ppm Cl-1.
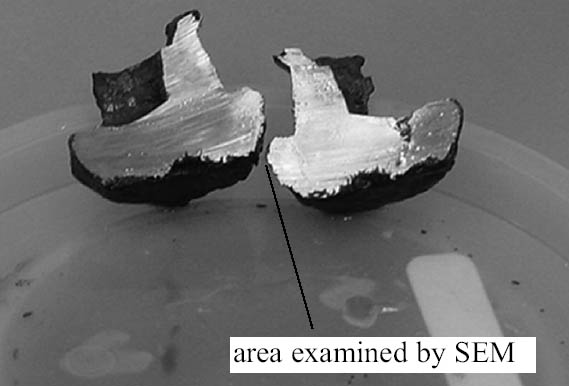
3.1 PRELIMINARY RIVET ANALYSISTo analyze the extent of corrosion prior to the extraction experiments, a special holder was designed and fabricated to hold the rivets to be evaluated for vertical sectioning using the diamond saw. In figures 2 and 3, images of rivet HL-1807 (stored in tap water) before and after cutting illustrate the overall condition of the surface and of the interior. As shown in figure 3, all the apparent corrosion appears to be confined primarily to the surface of the artifact. As part of this experiment, water displacement was used to estimate the density of the rivet. The estimated density was 5.2 g/cm3, which was approximately 70% of the density of a forged steel test specimen used for comparison. These density results were considered consistent with the visual appearance of
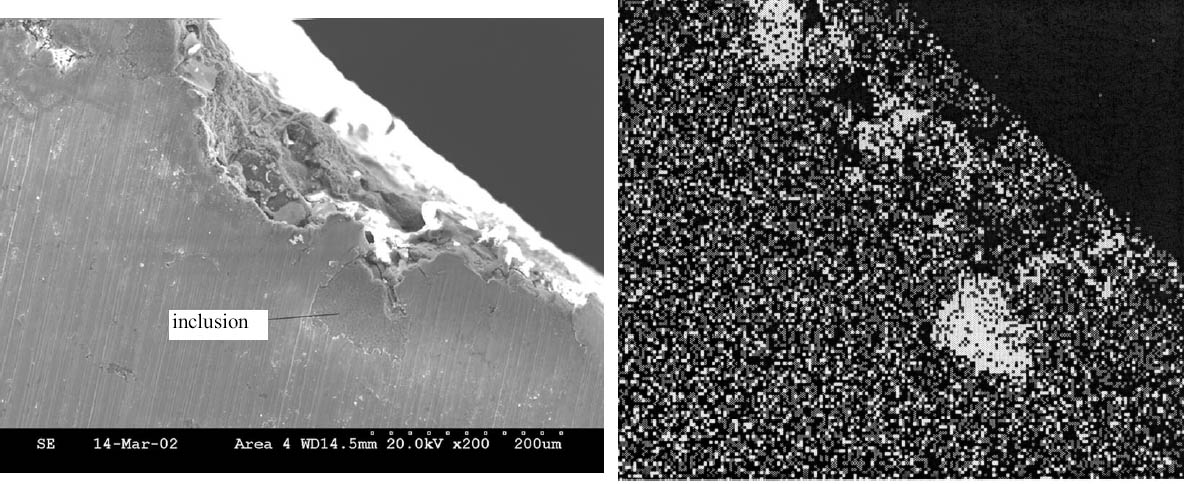
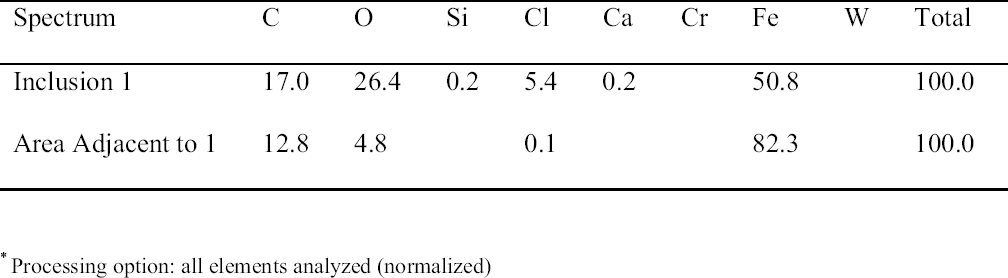
Rivet areas that were examined during these experiments included a remnant of the artifact that was on the outside of the hull where extensive corrosion was observed. The corrosion pattern observed in this area was consistent with patterns found in other areas of the rivet and was characterized by an irregular growth front with channels leading well below the average depth of the corrosion. Whether this pattern represented movement along grain boundaries has not yet been established. Somewhat surprisingly, only a few areas of high chlorine concentration were detected by EDX in these corroded regions. On the other hand, an area (identified in fig. 3) on the head of the rivet inside of the hull, which included an internal inclusion, displayed much higher levels of Cl. A micrograph of this region along with a Cl map is shown in figure 4, and the qualitative analysis results are summarized in table 2. In addition to the high levels of chlorine in this inclusion, as shown in figure 4, high levels were also observed along most of the corrosion front in this area. The corrosion products in the shavings were assumed to be similar to those on the rivet exterior because they were obtained from adjacent areas. It was also assumed that because of their relatively small size and high surface-to-volume ratios, as compared to an intact rivet, extraction from the rivet shavings would be more efficient. Finally, because of their different storage conditions, this set of samples could also represent what might happen if a recovered artifact were allowed to simply dry out or were stored in plain tap water instead of immersed in a caustic solution shortly after recovery. 3.2 DIGESTION OF SHAVINGS FROM SETS A, B, AND CThe principal samples used in this part of the study were the metal shavings generated when the rivets were removed by drilling from the hull plates of the H. L. Hunley prior to their removal for the excavation of the interior of the submarine. After their removal in February 2001, the rivets from one of the hull plates were stored in tap water and those from the other in 2 wt% NaOH solutions made up
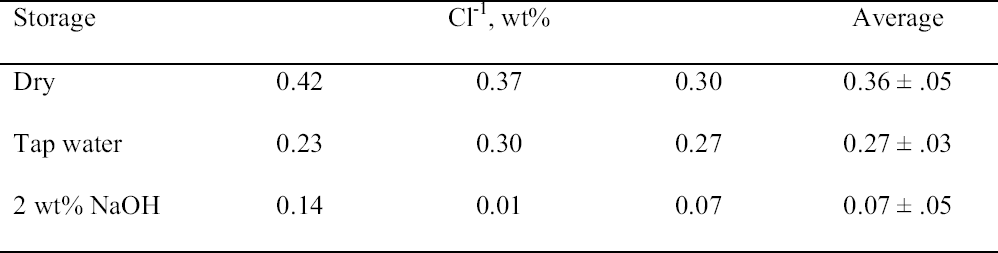
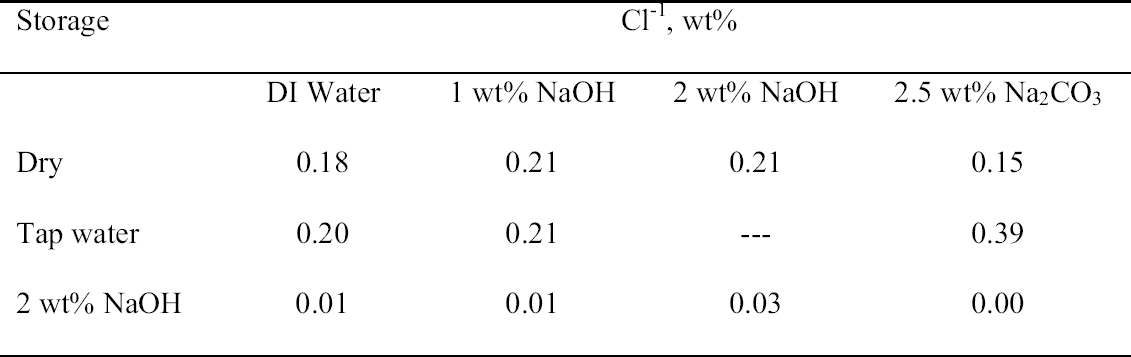
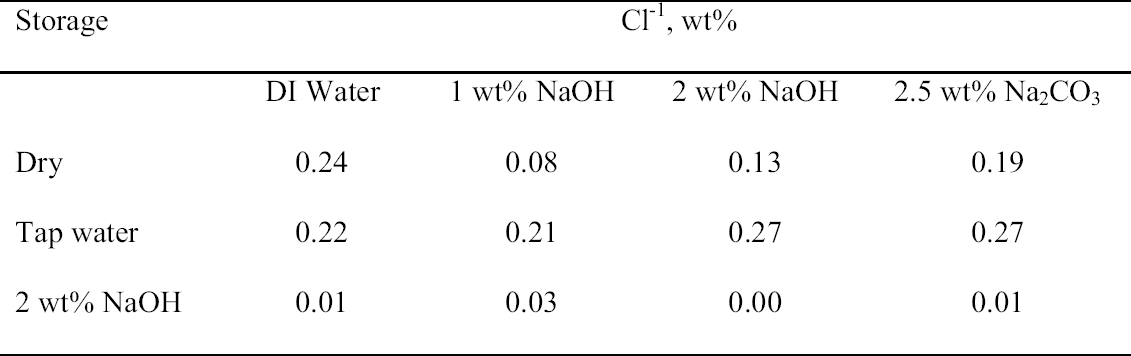
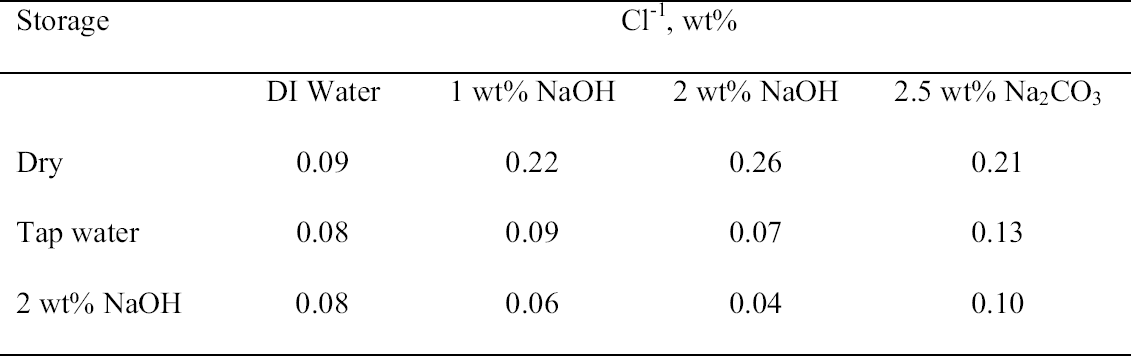
That the storage conditions had a significant effect on the physical appearance of the rivet shavings was immediately obvious, as shown by the photographs of representative samples from each set shown in figure 5. The shavings stored dry (set C) were reddish brown and contained a significant amount of powdery residue. The shavings stored in tap water (set A) were darker in color, and the storage solution contained a significant amount of suspended colloidal material. The shavings stored in 2 wt% NaOH (set B) were very dark, almost black, and the storage solution contained almost no suspended material and was very clear. Because of the heterogeneous nature of these shavings and their corrosion products, as illustrated by the samples shown in figure 5, for each of the digestion or extraction experiments special care was taken to select representative samples. The approach chosen to ensure representative sampling was to start with a much larger sample, distribute it evenly on a surface, and then randomly select subsamples of the appropriate size. Prior to carrying out the extraction experiments, the total weight percent Cl-1 content of each set of rivet shavings was determined by digestion. In the first series of digestion experiments, triplicate samples from each set were analyzed. The results are summarized in table 3. As clearly shown by these data, the storage conditions affected the Cl-1 content of the rivet shavings as well as their physical appearance. As expected, storage in 2 wt% NaOH (set B) had the most dramatic effect on both the physical appearance and Cl-1 content of the shavings, with a Cl-1 content at least 75% less than that measured for the other two conditions. To evaluate the reproducibility of the digestion and sampling procedures, as well as to obtain more statistical data to allow for a comparison of the dry- and tap-water–stored shavings, a second series of digestions with five replicates each was undertaken. The results from these experiments are summarized in table 4, and the averages and standard deviations from the two data sets are compared in figure 6. When the data in tables 3 and 4 are compared, it is obvious that the two wet samples (sets A and B) are the most difficult to sample reproducibly and generate
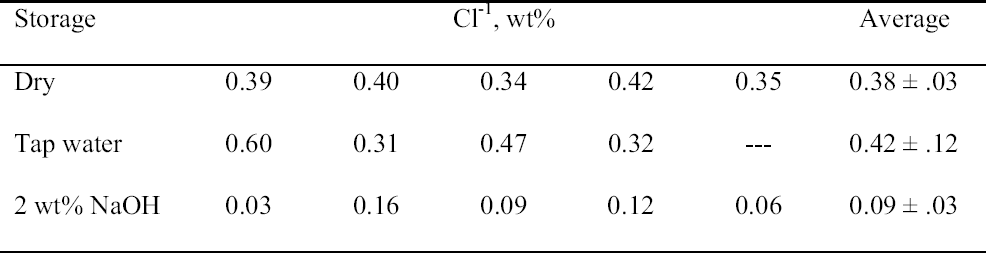
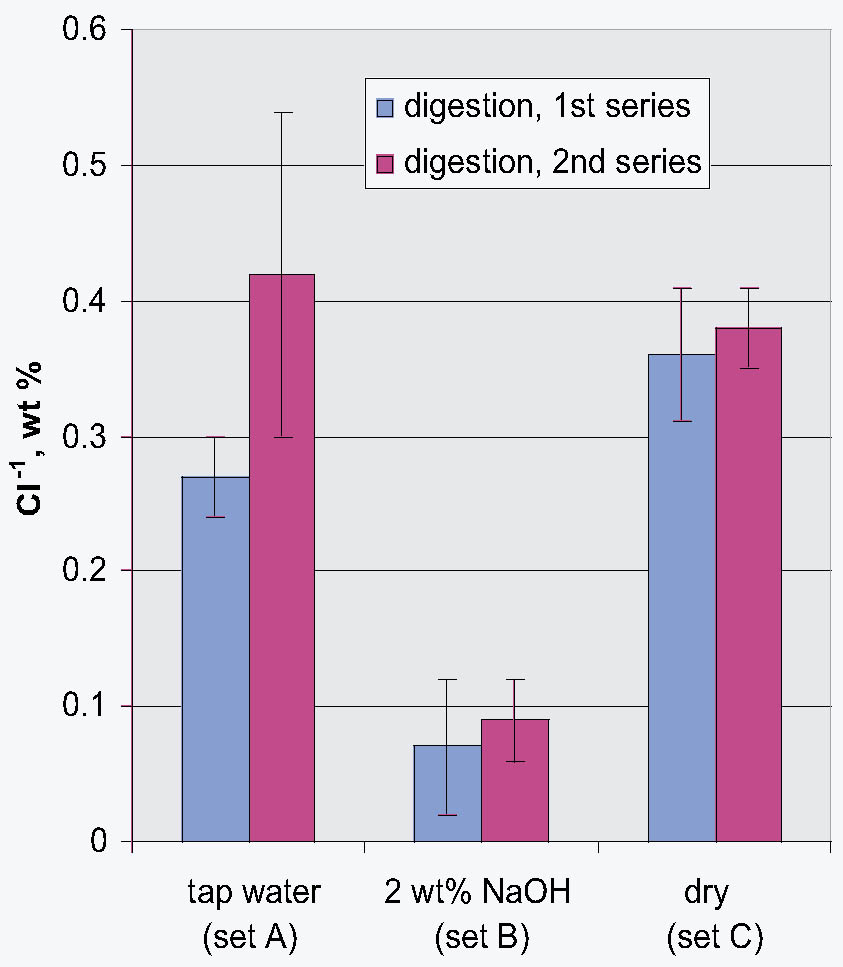
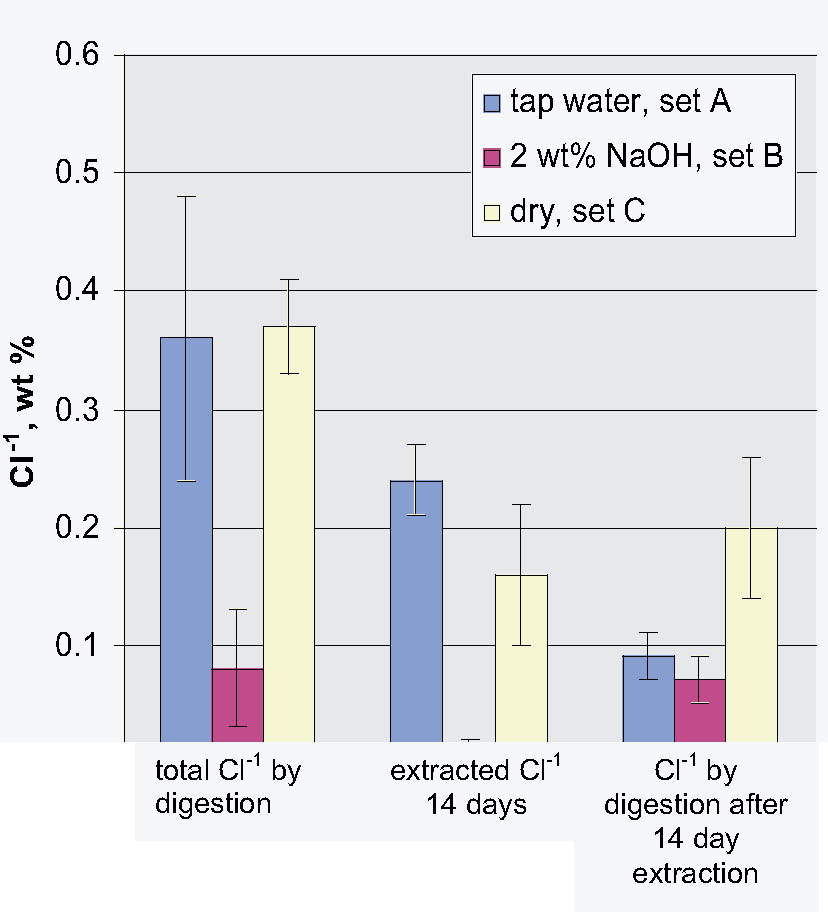
3.3 72-HOUR EXTRACTION OF SHAVINGS FROM SETS A, B, AND CIn the next sequence of experiments, rivet shavings selected and prepared as for the digestions were extracted using a variety of solutions in an attempt to characterize how easily removable the Cl-1 content was in each set. For the initial extraction experiments, an extraction time of 72 hours and DI water, 2.5 wt% Na2CO3 and 1 or 2 wt% NaOH in DI water solutions, were employed. The alkaline solutions were selected based on their frequent utilization in the conservation literature for the removal of Cl-1 from iron artifacts. The DI water was used for comparative purposes and to represent an extraction solution that would be expected to remove only relatively free Cl-1 without significant counterion replacement. The data for wt% Cl-1 extracted from the rivet shavings after 72 hours are summarized in table 5. When the results summarized in table 5 are compared to the digestion data, it is clear that except for the extraction of the rivet shavings stored in tap water with the 2.5 wt% Na2CO3, only ~ 50% of the Cl-1 was removed in these experiments. On the other hand, the Na2CO3 result may simply reflect the extraction of a high Cl-1 tap water sample, as the data from the set A digestion samples ranged from a low of 0.23 to a high of 0.60 wt% Cl-1. As with the digestion results, the extraction results are consistent with the conclusion that the Cl-1 content of the rivet shavings stored dry and stored in tap water (sets C and A) was the same and significantly higher than in the shavings stored in NaOH (set B). Surprisingly, there appeared to be no obvious difference in the extraction efficiencies of any of the solutions used. 3.4 14-DAY EXTRACTION FOLLOWED BY DIGESTION OF SHAVINGS FROM SETS A, B, AND CConsequently, a second set of extraction experiments using a time of 14 days, with and without stirring the samples, was performed. Since no difference was apparent in the extraction results for the samples that were agitated and those that were not, these data were combined, and these results are summarized in table 6. A comparison of the data in tables 5 and 6 clearly shows that, overall, the extraction efficiencies at 14 days were very similar to those observed at 72 hours. In fact, if the average Cl-1 extracted from the shavings stored dry and in tap water (sets C and A) is calculated, at 72 hours it was 0.22 � 0.07, and at 14 days it was 0.20 � 0.06 wt%, which are statistically the same. Similarly, for the shavings stored in NaOH (set B), there was no statistical difference between the two extraction times. It is also clear that even though the Cl-1 content of the rivet shavings stored in NaOH was significantly lower than in the other two samples, very little of this remaining chloride appears to be readily extractable. To compare the extraction data and the digestion results more directly, the solids remaining after the 14-day extraction were then digested, and these results are summarized in table 7. Perhaps the most interesting observation that can be made regarding the data in table 7 is that for the shavings stored in the two solutions, the wt% Cl-1 remaining in the shavings after extraction appears to be statistically almost the same: 0.09 � 0.02 (set A, tap water) and 0.07 � 0.02 (set B, NaOH) and significantly less than in those stored dry, 0.20 � 0.06 wt% (set C). To view these data from a different perspective, the data from tables 6 and 7 as well as the shaving digestion data from the three sets of shavings are compared in graphic format in figure 7. Presented in this manner, these results clearly show the effects of the changes in the rivet storage conditions on the total amount of readily extractable Cl-1. In addition, as might be expected, the dry shavings from set C appeared to contain the least amount of readily extractable Cl-1, and those stored in tap water from set A the most. Whether any additional Cl-1 could be removed by extracting for a longer period of time from any of these samples is uncertain at this time. Also, as is illustrated in figure 7, even after storage for a period of almost two years in 2 wt% caustic solution, a significant concentration (greater than 500 ppm) of very difficult to remove Cl-1 remained in the rivet shavings from set B. Whether this concentration represents Cl-1 that is chemically bound to a corrosion product, that is trapped as an inclusion in a corrosion product such as akagan�ite, or that is simply deep within the corrosion pore structure has yet to be determined.
4 SUMMARY AND CONCLUSIONSThis article addresses the issue of the presence of free and bound chloride in the metal shavings retained when the hull plate rivets were drilled as part of their removal process, prior to the excavation of the Hunley's interior. Before the extraction and digestion studies on the shavings were begun, one of the rivets that had been stored in tap water since its removal was sectioned. The sectioned surface was characterized using scanning electron microscopy and energy dispersive x-ray analysis. This analysis showed the general nature of the corrosion processes that had occurred in the rivet over nearly 140 years. Of particular interest was the presence of inclusions containing high levels of Cl-1 (> 50,000 ppm). Rivet shavings that had been stored dry, in tap water, and in 2 wt% NaOH immediately after removal were extracted with deionized water, 2.5 wt% Na2CO3, and 1 and 2 wt% NaOH solutions for up to two weeks. The total amount of chloride present in each sample was determined using nitric acid digestion. It was found that the shavings stored dry and in tap water contained very similar quantities of total chloride, ~ 0.4 wt%. It was also found that the shavings stored in caustic solution contained significantly less chloride than the other two sets of samples, less than 0.1 wt%. For all of the shaving samples, it was found that a significant quantity of chloride (> 500 ppm) remained in the corrosion products after extraction. Some of the more significant conclusions that may be drawn from these results are summarized below:
ACKNOWLEDGEMENTSThe authors would like to thank the Department of Defense (through the DOD Legacy funds), the state of South Carolina (Hunley Commission), and the Friends of the Hunley for supporting this research. We are especially indebted to Bob Neyland, Hunley Project director, Warren Lasch, chairman of the Friends of the Hunley, and Maria Jacobsen, senior archaeologist, for their support and to all the personnel of the Warren Lasch Conservation Center for their assistance. One of the authors would also like to especially thank Clemson's School of Materials Science and Engineering and the College of Engineering and Science for allowing his participation in this research as well as the partial financial support for this work received from Clemson University. We would like to thank Linda Jenkins of Clemson's Department of Bioengineering for her assistance in sectioning the rivet sample, Bill Kay of Clemson's Electron Microscope facility for the electron micrographs of the rivet sections, and Kim Ivey of the School of Materials Science and Engineering for her assistance in sample preparation. REFERENCESAl-Zahrani, A.1999. Chloride ion removal from archaeological iron and β-FeOOH. Ph. D. diss., University of Wales, Cardiff, U. K. Gilberg, M. R., and N. J.Seeley. 1981. The identity of compounds containing chloride ions in marine iron corrosion products: A critical review. Studies in Conservation26:50–56. Kaneko, K.1989. Surface chemistry of FeOOH microcrystals. In Current problems in the conservation of metal antiquities. 13th International Symposium on the Conservation and Restoration of Cultural Property, Tokyo, Japan. Tokyo: National Research Institute of Cultural Properties. 42–62. Knight, B.1982. Why do some iron objects break up in storage? In Conservation of iron,ed. R. W.Black and S. M.Blackshaw. Maritime Monographs and Reports 53. London: Greenwich Trustees of the National Maritime Museum. 50–55. North, N. A.1982. Corrosion products on marine iron. Studies in Conservation27:75–83. North, N. A., and C.Pearson. 1978a. Methods for treating marine iron. ICOM Committee for Conservation preprints. 5th Triennial Meeting, Zagreb. Paris: ICOM. 1–10. North, N. A., and C.Pearson. 1978b. Washing methods for chloride removal from marine iron artifacts. Studies in Conservation23:174–86. Rinuy, A., and F.Schweizer. 1981. M�thodes de conservation d'objets de fouilles en fer: Etude quantitative compar�e de l'�limination des chlorures. Studies in Conservation26:29–41. Selwyn, L. S.1999. Corrosion of archaeological iron before and after excavation. Proceedings of the Northern Area Eastern Conference and Exhibition. NACE International Paper 2B. 1.Ontario, Canada: CCI. 1–8. Selwyn, L. S., W. R.McKinnon, and V.Argyropoulos. 2001. Models for chloride ion diffusion in archaeological iron. Studies in Conservation46:109–20.
Stalh, K., K.Nielsen, J. C.Hanson, P.Norby, J. Z.Jiang, and J.Van Lanschot. 1998. The akaganeite-hematite reaction on the possibilities for chloride removal from iron artifacts. School of Conservation Jubilee Symposium preprints.Copenhagen: Konservatorskolen Det Kongelige Danske Kunstakademi. 157–60. Turgoose, S.1982a. The nature of surviving iron objects. In Conservation of iron,ed. R. W.Black and S. M.Blackshaw. Maritime Monographs and Reports 53. London: Greenwich Trustees of the National Maritime Museum. 1–7. Turgoose, S.1982b. Post-excavation changes in iron antiquities. Studies in Conservation27:97–101. Yabuki, H., and M.Shima. 1979. Akaganeite as an oxidized product of iron implements from the vestiges of ancient age. Scientific Papers of the Institute of Physical and Chemical Research73:71–78. AUTHOR INFORMATIONN�STOR G. GONZ�LEZ received a degree in chemistry in 1996 and graduated in 2002 in chemical engineering from the Universidad de la Republica in Uruguay. He worked with the underwater archaeological team at the Uruguayan National Heritage Office (Comision del Patrimonio Cultural de la Nacion). He is currently working as a chemical engineer on the H. L. Hunley Project. His research interests include the corrosion and conservation of metals as well as nonmetallic artifacts recovered from marine environments. Address: H. L. Hunley Project, Warren Lasch Conservation Center, 1250 Supply Street Bldg. 255, North Charleston, S.C. 29405 PHILIPPE DE VIVI�S graduated in 1992 as a metallurgist specializing in heat treatment processes. He began to work in the field of underwater conservation with Paul Mardikian in 1995. In August 2000, he joined the H. L. Hunley Project to work on his master's degree in the conservation of cultural properties from the Paris I Pantheon Sorbonne University conservation program. He is currently working as a conservator at the Warren Lasch Conservation Center in Charleston, South Carolina. De Vivi�s's experience includes work in field conservation and treatment of artifacts from the CSS Alabama and a number of ancient shipwrecks. He has also worked in Alexandria, Egypt, with Jean-Yves Empereur, director of research at the Centre National de la Recherche Scientifique (CNRS) in collaboration with the French Institute of Oriental Archaeology. Address as for Gonz�lez. MICHAEL J. DREWS received his B.S. degree in chemistry from the University of Wisconsin at Madison and his Ph.D. in physical chemistry from the University of North Texas. He was a postdoctoral student under Dr. Robert H. Barker at Clemson University. He has also been a guest worker at the Center for Fire Research at the National Institute for Science and Technology and is currently on sabbatical at the Warren Lasch Conservation Center in Charleston, South Carolina. His research interests include the corrosion and conservation of iron artifacts as well as the use of dense gas fluids in the conservation of nonmetallic artifacts from marine sites. Address as for Gonz�lez. PAUL MARDIKIAN obtained his M.A. in archaeology and art history from the School of the Louvre in Paris in 1989 and his M.S. in conservation sciences and techniques from the Sorbonne University in 1991. Specializing in marine archaeological conservation, he worked several years with the team from the Electricit� de France Valectra Laboratory on the conservation of artifacts from the Titanic, with specialists from the Canadian Heritage Parks Canada in the area of wet organic materials and composites. He joined the team of the Western Australian Maritime Museum as an honorary research fellow. His experience in the field of maritime archaeology and conservation ranges from ancient shipwrecks in the Mediterranean to the conservation of American Civil War artifacts. His work includes the conservation for the U.S. Navy of numerous artifacts retrieved from the Confederate raider Alabama sunk off France in 1864. He has been conserving artifacts for the Naval Historical Center in Washington, D.C., since 1988, and he has been serving as senior conservator on the H. L. Hunley Project since 1999. Address as for Gonz�lez.
 Section Index Section Index |