THE EFFECT OF SUPERCRITICAL CARBON DIOXIDE EXTRACTION ON COLOR RETENTION AND PESTICIDE REDUCTION OF WOODEN ARTIFACTSSUNG MO KANG, ACHIM UNGER, & J.J. MORRELL
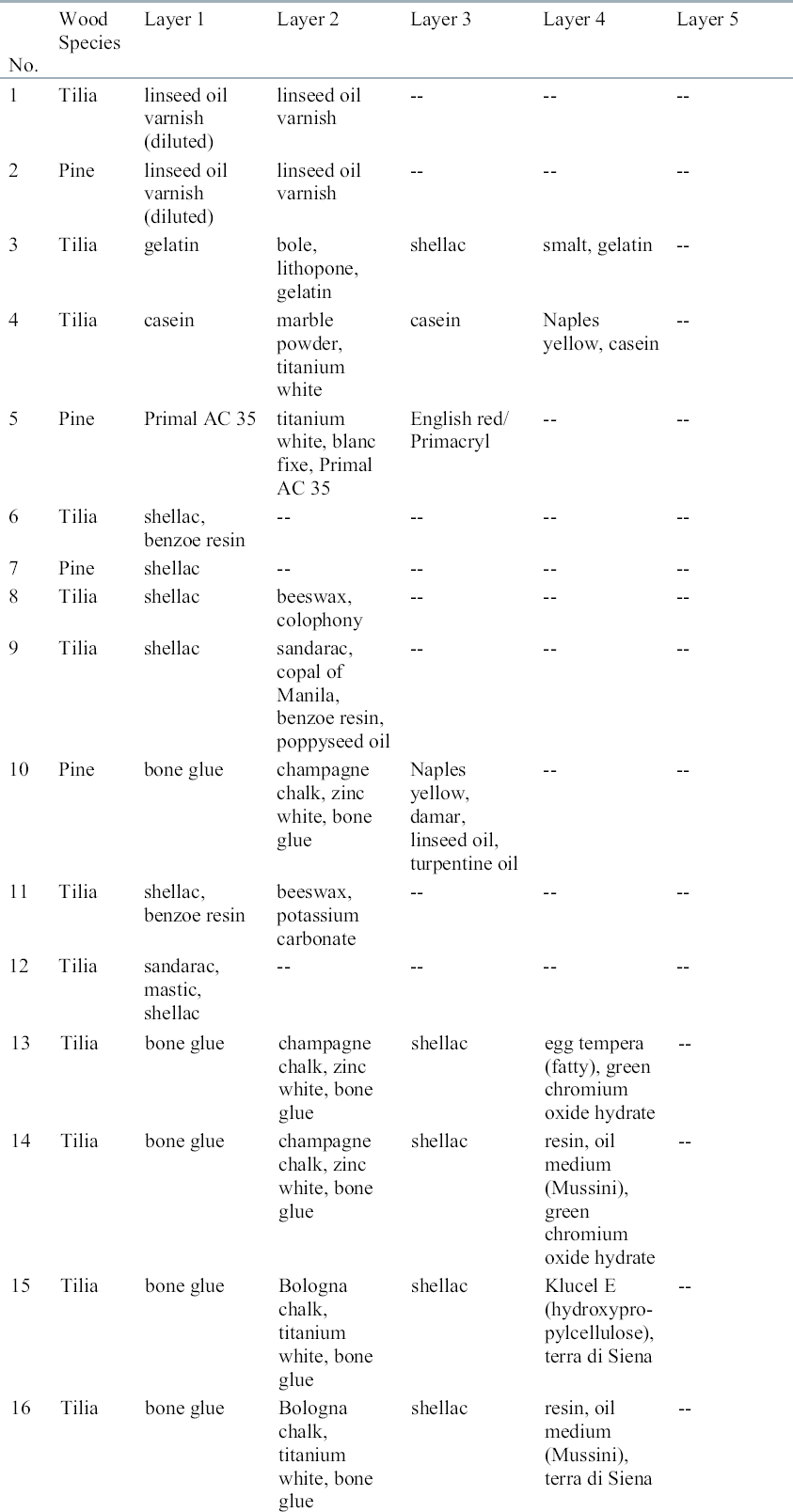
ABSTRACT—The effects of supercritical fluid carbon dioxide extraction on the luminosity of various pigments used to decorate wooden artifacts was assessed as a prelude to using this treatment technology for removing pesticides—specifically DDT—from wooden artifacts. The extraction darkened 6 of the 31 coatings evaluated and lightened 9 others. The color changes were slight and often difficult to detect visually. The remainder of coatings experienced no significant changes. DDT was extracted from all blocks regardless of coating combinations. DDT reductions of 50% or more were observed with 26 of 31 coating combinations, suggesting that this treatment may be used for pesticide extraction without adversely affecting wooden artifacts. TITRE—Les effets de l'extraction par gaz carbonique � l'�tat supercritique sur des objets en bois: la r�tention des couleurs et l'enl�vement des pesticides. R�SUM�—Les effets de l'extraction par du gaz carbonique liquide � l'�tat supercritique sur la luminosit� de divers colorants employ�s pour d�corer les objets en bois ont �t� �valu�s, pr�alablement � l'utilisation de cette technique pour extraire des pesticides, et sp�cifiquement le DDT, de ces objets. Un certain assombrissement a �t� not� dans le cas de 6 des 31 enduits �valu�s, tandis que 9 autres enduits sont devenus plus clairs. Les changements de couleur �taient l�gers et souvent difficiles � d�tecter � l'oeil nu. Le traitement n'a produit aucun changement notable pour les autres enduits. Le DDT a �t� extrait � partir de tous les sp�cimens, ind�pendamment des types d'enduits. On a observ� des r�ductions du pourcentage de DDT de 50% ou plus pour 26 de 31 types d'enduits, sugg�rant que ce traitement peut �tre employ� pour l'extraction des pesticides sans compromettre les objets en bois. TITULO—Efecto de la extracci�n con dioxido de carbono supercr�tico en la retenci�n del color y en la reducci�n de pesticidas en artefactos de madera. RESUMEN—Se estudiaron los efectos de la extracci�n con di�xido de carbono como fluido supercr�tico sobre la luminosidad de varios pigmentos utilizados para decorar artefactos de madera con el prop�sito de usar este tratamiento en la eliminaci�n de pesticidas, espec�ficamente DDT, de artefactos de madera. La extracci�n oscureci� 6 de los 31 recubrimientos evaluados y aclar� 9 de ellos. Los cambios de color fueron leves y a menudo dif�ciles de detectar visualmente. Los recubrimientos remanentes no experimentaron cambios significativos. Se extrajo DDT de todos los bloques independientemente de las combinaciones de recubrimientos. Se observaron reducciones de DDT del 50% o superiores en 26 de las 31 combinaciones de recubrimientos, lo cual sugiere que este tratamiento puede ser utilizado en la extracci�n de pesticidas sin afectar de manera adversa los artefactos de madera. T�TULO—O efeito da extra��o por di�xido de carbono supercr�tico na reten��o de cores e na redu��o de pesticida em artefatos de madeira. RESUMO—Os efeitos da extrac�o por di�xido de carbono suprecr�tico fluido na luminosidade de v�rios pigmentos usados para decorar artefatos de madeira foram avaliados como pre�mbulo ao uso desta tecnologia de tratamento na remo��o de pesticidas–especificamente o DDT – dos artefatos de madeira. A extra��o escureceu 6 dos 31 revestimentos que foram avaliados e clareou 9. As mudan�as de cor foram insignificantes e, com frequ�ncia, dif�ceis de serem detectadas visualmente. Os demais revestimentos n�o apresentaram mudan�as significativas. O DDT foi extra�do de todos os blocos, independentemente das combina��es de revestimentos. Redu��es de DDT da ordem de 50% ou mais foram observadas em 26 das 31 combina��es, sugerindo que este tratamento pode ser utilizado para extra��o de pesticida sem afetar os artefatos em madeira de modo indesej�vel. 1 INTRODUCTIONFor centuries, religious artifacts such as icons have been carved from indigenous materials, including DDT was generally applied in solvent-based formulations that penetrated to various depths into the wood, depending upon the wood species. The primary goal of its use was preventive rather than remedial, and most of the preservative was confined near the surface. Later, the safe handling of DDT-treated objects became a matter of concern, as did the potential for adverse effects when DDT bloomed on the wood surface. While DDT can be extracted using conventional solvents, these solvents can be damaging to the artifacts or the pigments used to color or decorate them. Many wooden artifacts were coated with a variety of pigments and sealants in specific sequence to create certain hues. The coatings consist of both organic and inorganic compounds. Extraction with organic solvents can selectively remove pigment components, producing deleterious changes in both color and coating adhesion. One alternative to conventional solvent extraction could be the use of supercritical fluids (SCF). SCFs have the ability to penetrate materials like a gas, thus offering the potential for easily penetrating a material or the film coating on the surface to strip away residual pesticide. The other advantage of using SCFs is that solubility is infinitely adjustable by varying either temperature or pressure within the critical region (Clifford 1998). These properties have encouraged the use of SCFs to recover natural products from a variety of wood-based materials (Fremont 1981; Larsen et al. 1992; Terauchi et al. 1993; Ohira et al. 1994). SCFs solubilize materials like a liquid solvent, but it may be possible to adjust conditions so that DDT is solubilized without affecting pigment components. DDT can be readily extracted from contaminated soils using supercritical (SC) carbon dioxide (Snyder et al. 1992, 1993). There is relatively little literature, however, on either the ability of SCF to extract pesticides from wood or the effects of extraction on the integrity of the artifact or surface coatings. Sahle-Demessie et al. (1997) successfully extracted pentachlorophenol from wood in work that led to two patents (Levien et al. 1994; Ruddick and Cui 1995). More recent studies have explored extraction of other pesticides, but DDT has been infrequently used for wood protection. SCF extraction of DDT from soil using carbon dioxide with or without a co-solvent appears to be relatively straightforward, suggesting that similar processes might be useful for remediating pesticide-contaminated wood artifacts (Snyder et al. 1993). While DDT may be easily extracted using SCFs, the process may have deleterious effects on the artifacts. We explored the potential effects of SCF extraction on the color of various simulated artifacts and its ability to remove DDT. 2 MATERIALS AND METHODSLimewood (Tilia sp.) or pine sapwood (Pinus sylvestris) samples (15 � 25 by 50 mm) were coated with 1 of 31 different coating combinations representing materials normally used to paint wood icons (table 1). The coatings included both organic and inorganic materials. These materials were treated using Hylotox 59 containing 3.5% technical DDT and 0.5% lindane. Technical DDT consisted of about 30% 2, 4- and about 70% 4, 4′-isomers. The solvent mixture of Hylotox 59 was a petroleum distillate designated as Laval 300 (~ 95%), isobornyl acetate (0.5%), and turpentine oil (0.25%). Laval 300 included aliphatic hydrocarbons, aromatic hydrocarbons
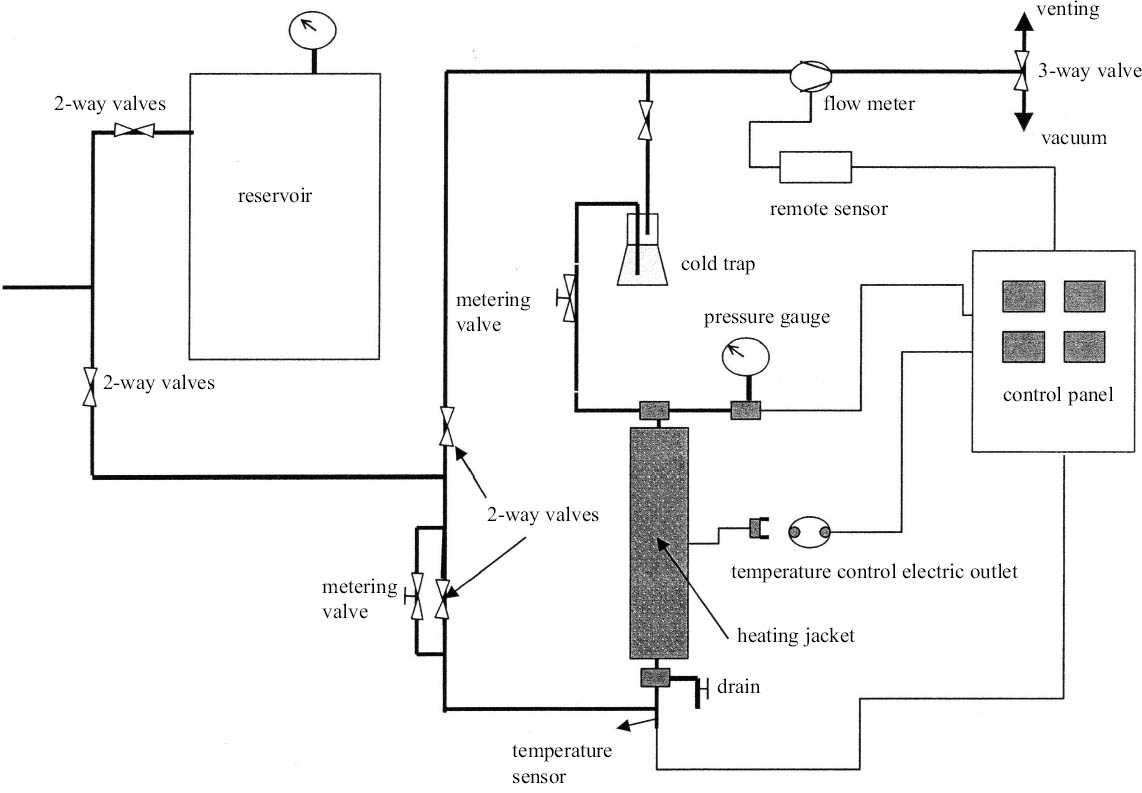
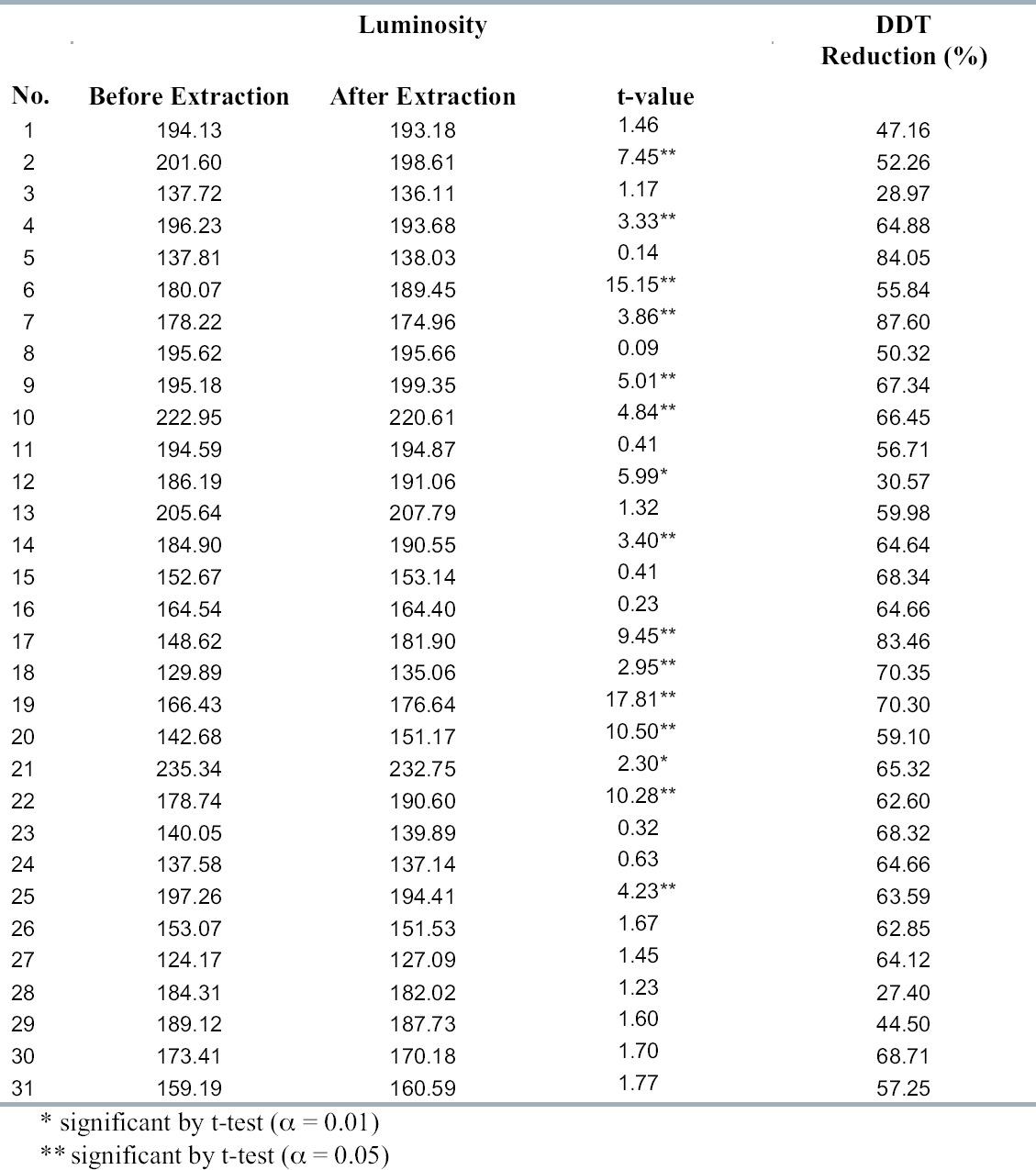
The potential for color change during processing was assessed by scanning specimens before and after treatment. Four specimens were used for each coating combination. The specimens were subjected to image analysis prior to treatment. Two wide faces of each sample were scanned using a digital scanner (600 dpi); then the entire scanned images were analyzed using Photoshop for luminosity and red, green, and blue color levels, which were measured on a scale of 0 to 255. The specimens were rescanned after treatment using the same scanner at the same settings, and the results were compared for each specimen. The samples were placed in an SCF extraction device consisting of a 90.8 mm inner diameter by 1036 mm long vessel preheated to 45�C (fig. 1). Carbon dioxide was admitted at a pressurization rate of 10 psi/second until pressure reached 3000 psi. The samples were maintained at 3000 psi under static conditions for 10 minutes. Then carbon dioxide was flowed through the vessel at 94.4 ml/minute for an additional 20 minutes. The vessel was then depressurized at 500 psi/minute. Following treatment, the two wide faces of each sample were scanned again and the results of the pre- and posttreatment scans were averaged for the four specimens per coating. These means were compared using t-tests at α = 0.01 or 0.05. The extracted samples were cut into two equal pieces. Then each sample was ground to pass a 20-mesh screen. The ground wood was thoroughly mixed, and 0.15 g of ground wood was mixed with 10 ml of methanol. The mixture was sonicated for three hours at 65�C. DDT concentrations in the extracts were determined by injecting one μl into a Shimadzu high-resolution gas chromatograph with a low-resolution mass spectrometer. Separation was achieved using a XTI-5 capillary column (Restek) (0.25 mm inside diameter � 30 m long) composed of fused silica with a 0.25 μ thick film of 95% dimethyl, 6 diphenyl polysilarylene. The carrier gas was Grade 5 helium at flow and split rates of 1.2 ml/minute and 5, respectively. Injector and detector temperatures were 250�C and 280�C, respectively. After holding for two minutes after injection, column temperature was increased from 35�C to 260�C at 25�C/minute. DDT levels were quantified by comparison with known standards. The residual DDT was calculated by subtracting the analyzed retention from the net initial retention. 3 RESULTS AND DISCUSSIONScanning data were expressed on the basis of luminosity and red, green, or blue intensity. All four measurements appeared to be affected by extraction, but luminosity appeared to be the most consistently affected. Luminosity significantly changed in 15 of 31 treatments, with 6 treatments darkening, 9 treatments lightening, and the remainder unchanged (table 2). In most cases, the degree of color change, while significant, was difficult to detect with the naked eye. The relatively small changes in luminosity following extraction suggest that color changes might be further minimized using slight process variations to alter the solubility of specific pigment components. The presence of specific components in the coatings might be expected to render a material more or less susceptible to SCF extraction. SC carbon dioxide tends to work best with nonpolar materials because its polarity is lower than any hydrocarbon except All treatments with Naples yellow (nos. 4, 10, and 25) became darker after SCF extraction, suggesting this pigment might react with SC carbon dioxide during the process. A total of 20 coatings had a shellac component, and 9 of these turned significantly lighter with treatment. Shellac is an organic compound and should be very susceptible to SC carbon dioxide. Two other shellac-containing coatings experienced significant darkening, while the remaining 9 coatings were unaffected. Similarly, 14 coatings contained bone glue as their base, but only 4 of these experienced significant changes in luminosity. The presence of a specific component did not appear to consistently influence changes in luminosity. The results suggest that minor changes in coating can affect susceptibility to SC carbon dioxide, and careful testing of a coating will be essential prior to SCF extraction to ensure that it is not negatively affected by treatment. SCF easily extracted DDT from all specimens regardless of coating combinations, producing an average extraction efficacy of 60%. DDT levels declined by at least 50% of the initial retention in 26 of 31 coating treatments and about 45% in 2 others. DDT reductions were about 30% for 3 coating combinations (see table 2). SCF extraction solubilized substantial quantities of the chemical from within the sample. Longer extraction times would be expected to produce higher DDT reductions, as could higher pressures or the addition of co-solvents. One of the advantages of SCF extraction is the infinite adjustability of temperature and pressure conditions
Coating type did not appear to affect extraction efficiency, although some blocks were less thoroughly extracted. The reason for a lower degree of extraction with some coating combinations is difficult to determine. For example, three coatings contained bone glue followed by skin glue, stone chalk, champagne chalk, Bologna chalk, and shellac, followed by a final layer that varied by treatment. While the colors of all three samples did not differ significantly after SCF extraction, DDT reductions were 27.4%, 44.5%, and 68.71% of the initial DDT retentions, respectively.
Limewood and pine sapwood receiving the same coatings plus DDT were used to determine the effect of wood species on extraction. Each sample receiving the identical coating (two coats of linseed oil varnish) experienced similar % DDT reductions (see tables 1 and 2). Limewood and pine sapwood are both very permeable species, with permeabilities of 104 and 103cm3 (air)/(cm atm second), respectively (Siau 1971). Permeability can influence the penetration of super-critical fluid into wood. Schneider (1999) measured time-to-pressure equilibrium in 30 � 30 � 60 mm samples after vessel pressure reached 10.3 Mpa for pine sapwood, Douglas fir heartwood, spruce heart-wood, and red cedar heartwood. While the pressure equilibrated immediately in permeable species like pine, significantly extended time periods were required for the pressure equilibrium in Douglas fir, red cedar, and spruce heartwood. While SCF extraction was able to remove DDT from permeable species such as pine or limewood with minimum color alteration, further studies will be required to investigate extraction of impermeable species. Although SCF extraction successfully removed DDT from coated wood artifacts, our results clearly indicate that the extraction will require more systematic studies to understand how various coatings affect extraction efficiency and to minimize color change during the extraction. One advantage of SCF extraction is the ability to infinitely vary pressure or temperature and to employ modifiers to enhance the solubility of specific components or reduce that of others. The use of longer treatment cycles, alternative pressure and/or temperature conditions, and the addition of co-solvents would need to be explored to improve extraction efficiency without adversely affecting coating condition. 4 CONCLUSIONSSupercritical carbon dioxide extraction removed an average 60% of the original DDT from panels coated with pigments used on wooden icons, with minimal impact on the color quality of the pigments. While further studies will be necessary to refine extraction conditions and identify pigment components that are most susceptible to extraction conditions, the results indicate that SCF extraction may represent a promising method for nondestructively remediating pesticide-contaminated wooden artifacts. ACKNOWLEDGEMENTSThe authors wish to thank Hiltrud Fritsche, sculpture restorer in Berlin, for the preparation of the test specimens. REFERENCESClifford, T.1998. Fundamentals of supercritical fluids. Oxford: Oxford Science Publications. Fremont, H. A.1981. Extraction of coniferous wood with fluid CO2 or other supercritical fluids. U. S. Patent 4, 308, 200. Larsen, A., N.Jentoft, and T.Greibrokk. 1992. Extraction of formaldehyde from particleboard with supercritical carbon dioxide. Forest Products Journal42(4):45–48. Levien, K. L., J. J.Morrell, S.Kumar, and E.SahleDemessie. 1994. Process for removing chemical preservatives from wood using supercritical fluid extraction. U. S. Patent 5, 364, 475. Ohira, T., F.Terauchi, and M.Yatagai. 1994. Tropolones extracted from the wood of western red cedar by supercritical carbon dioxide. Holzforschung48:308–12. Ruddick, J. N. R., and F.Cui. 1995. Extraction of toxic organic contaminants from wood and photodegradation of toxic organic contaminants. U. S. Patent 5, 476, 975.
Sahle-Demessie, E.1994. Deposition of chemicals in semi-porous solids using supercritical fluid carriers. Ph. D. diss., Oregon State University, Corvallis. Sahle-Demessie, E., J. S.Yi, K. L.Levien, and J. J.Morrell. 1997. Supercritical fluid extraction of pentachlorophenol from pressure-treated wood. Separation Science and Technology32(6):1067–85. Schneider, P. F.1999. Pressure measurement in wood as a method to understand pressure impregnation process: Bethell, Rueping, Lowry and supercritical carbon dioxide. Ph. D. diss., Oregon State University, Corvallis. Siau, J. F.1971. Flow in wood.Syracuse, N. Y.: Syracuse University Press. Snyder, J. L., R. L.Grob, M. E.McNally, and T. S.Oostdyk. 1992. Comparison of supercritical fluid extraction with classical sonication and soxhlet extraction for selected pesticides. Analytical Chemistry64:1940–46. Snyder, J. L., R. L.Grob, M. E.McNally, and T. S.Oostdyk. 1993. The effect of instrumental parameters and soil matrix on the recovery of organochlorine and organophosphate pesticides from soils using supercritical fluid extraction. Journal of Chromatographic Science31:183–91. Terauchi, F., T.Ohira, M.Yatagi, T.Ohgama, H.Aoki, and T.Suzuki. 1993. Extraction of volatile compounds from coniferous woods with supercritical carbon dioxide. Mokuzai Gakkaishi39(12):1421–30. Unger, A.1998. Umweltsch�dliche Holzschutzmittel. Restauro104(3):186–91. SOURCES OF MATERIALSAzurite, Bologna chalk, bone glue, canvas, champagne chalk, Cremnitz white, cinnabar, colophony, gold lacquer, Klucel E, lapis lazuli, mastic, Naples yellow, poppyseed oil, Primal AC 35, red lead, sandarac, shellac, skin glue, smalt, stone chalk, titanium white, turpentine oil, zinc whiteDr. Georg Kremer Farbm�hle D-88317 Aichstetten/Allg�u Germany Blanc fixe, copal of Manila, damar, benzoe, linseed oil, lithopones, marble powder, tow, potassium carbonate, terra di SienaFirma Propolis Oranienstra�e 14a D-10997 Berlin Germany Green chromium oxide hydrate, English red, gold ocher, mixtion (for leaf gold and leaf silver), Primacryl, resin/oil medium (Mussini), ultramarine blue, ultramarine blue (light)Firma H. Schmincke & Co. Otto-Hahn-Str. 2 D-40699 Erkrath Germany Rembrandt oil paints with Naples yellow (table 1, no. 10), cinnabar (table 1, no. 18) and Cremnitz white (table 1, no. 21)Royal Talens B.V. Postbus 4 NL-7300 AA Appeldoorn/Holland The Netherlands Ammonia casein, beeswax, casein, gelatin, leaf gold (citron gold, ducat gold), leaf silver, rabbit skin glue, red bole, white boleFirma W. Wasner, Blattgold GmbH Wallenrodstr. 8–10 D-91126 Schwabach Germany AUTHOR INFORMATIONSUNG-MO KANG received his M.Sc. and Ph.D. degrees in wood science from Oregon State University, Corvallis, Oregon, in 1998 and 2002, respectively. He is a research associate in the Department of Forest Products at Korea Forest Research Institute in the Republic of Korea. His research interests include stain fungi and their control, preservative treatment of refractory species, supercritical fluid biocide impregnation and extraction of wood-based materials, and the migration of preservatives from treated wood. Address: Korea Forest Research Institute, 207 Cheongnyangni-Dong, Dongdaemun-Gu, Seoul, 130-712, Republic of Korea ACHIM UNGER received a Ph.D. in natural sciences from the Academy of Agricultural Sciences in Berlin, Germany. At present he is senior chemist in the Rathgen-Forschungslabor, Staatliche Museen zu Berlin. His research focuses on the analytical investigation of art objects made from organic materials, wood consolidants, pest control, and decontamination of biocides. He is co-author of Conservation of Wood Artifacts (2001). Address: Rathgen-Forschungslabor, Staaliche Museen zu Berlin, Preussischer Kulturbesitz, Berlin, Germany JEFFREY J. MORRELL received his B.S. and Ph.D. in forest pathology from the State University of New York College of Environmental Science and Forestry and his M.S. in plant pathology from Penn State. He is currently a professor in the Department of Wood Science and Engineering at Oregon State University. His research interests include aspects of biodeterioration and its prevention. Address: Department of Wood Science and Engineering, 119 Richardson Hall, Corvallis, Ore. 97333
 Section Index Section Index |