LITERATURE REVIEW: THE USE OF PARALOID B-72 AS A SURFACE CONSOLIDANT FOR STAINED GLASSSASHA CHAPMAN, & DAVID MASON
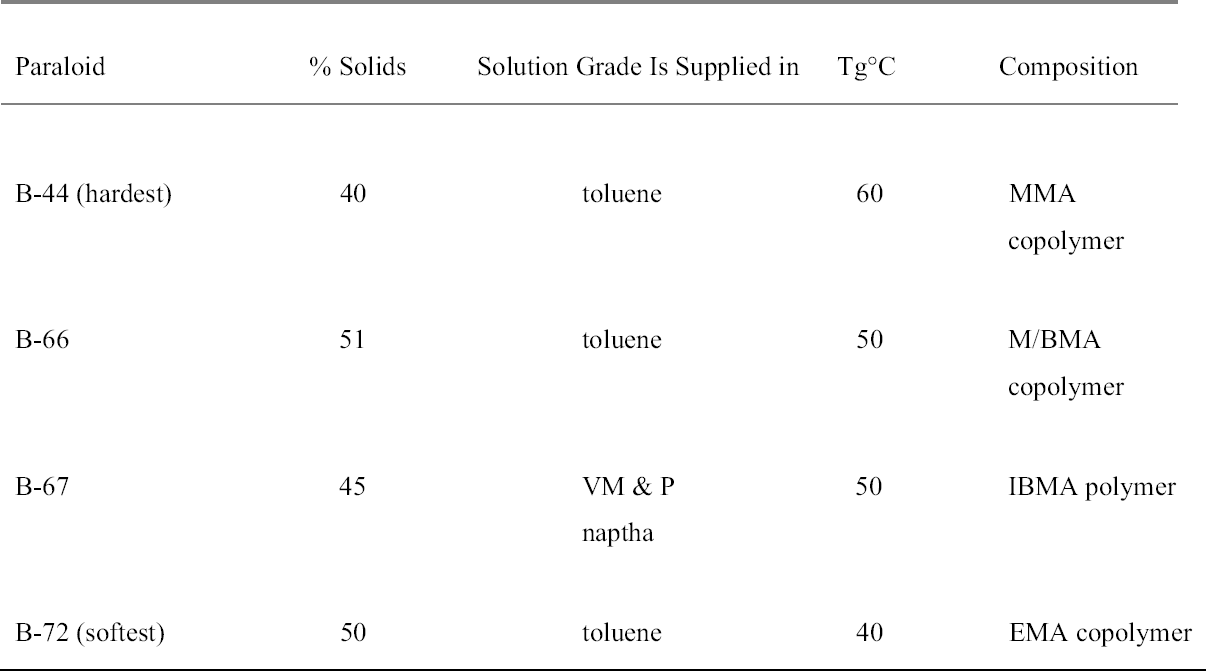
ABSTRACT—This paper was originally commissioned by English Heritage, the government's statutory adviser on archaeology and the historic environment in England, in the mid-1990s following concerns over the suitability of Paraloid B-72 for consolidation of loose paint in stained glass conservation. Paraloid B-72 has regularly been utilized for the re-adhesion of loose paint. But in spite of its many advantages (ease of use, reversibility, good aging properties, stability, and miscibility with several types of solvent), legitimate questions have been raised over its limitations (poor resistance to humidity, relatively weak bond with vitreous substrates, and solvent hazards).This article begins with a description of the chemistry and characteristics of Paraloid B-72 and its application in the conservation of historic painted glass. There follows a review of the current state of research on and practical experiences in the use of Paraloid B-72 and related systems. The article incorporates a summary of the known and tested properties of Paraloid B-72 in comparison with alternative materials currently under scrutiny for use in stained glass conservation and discusses the longer-term effects of treatment and the need for continued assessment of their efficacy. TITRE—Une revue de la litt�rature concernant l'utilisation du Paraloid B-72 en tant que consolidantde surface pour le vitrail. R�SUM�—Cet article a d'abord �t� command� au milieu des ann�es 1990 par English Heritage (la commission britannique sur les �difices et monuments historiques) qui sert comme conseiller statutaire pour le government sur l'arch�ologie et l'environnement historique enAngleterre. La raison �tait que des r�servations avaient �t� soulev�es au sujet de l'utilisation du Paraloid B-72 lors de la consolidation des couches picturales sur les vitraux. Le Paraloid B-72 est r�guli�rement utilis� pour consolider les couches picturales qui se soul�vent. Mais malgr� ses nombreux avantages (facilit� d'utilisation, r�versibilit�, bonnes propri�t�s de vieillissement, stabilit�, et solubilit� dans plusieurstypes de solvant), des questions l�gitimes ont �t� soulev�es � propos de certains de ses points faibles (pi�tre r�sistance � l'humidit�, faible adh�rence aux substrats vitreux et risques caus�s par les solvants).Cet article d�bute avec une description de la chimie et des caract�ristiques du Paraloid B-72 et de son application dans la conservation du verre historique peint. On y trouve ensuite une revue de l'�tat actuel de la recherche sur l'utilisation duParaloid B-72 et de syst�mes semblables, et des traitements couramment pratiqu�s. L'article pr�sente aussi une synth�se des propri�t�s actuellement connues et test�es du Paraloid B-72 et les compare avec celles d'autres mat�riaux qui font pr�sentement l'objet d'examens pour leur utilisation dans letraitement du vitrail. L'article se termine avec une discussion des effets � long terme du traitement et souligne le besoin de faire des suivis pour v�rifier si les traitements demeurent efficaces au fil du temps. TITULO—Revisi�n de literatura: El uso de Paraloid B-72 como un consolidante de superficie para el vidrio de color. RESUMEN—Esta investigaci�n fue encargada originalmente por la English Heritage a mediados de la d�cada de los a�os 1990, a causa de la preocupaci�n existente sobre si es adecuado o no el uso de Paraloid B-72 como un consolidante de pintura suelta en la conservaci�n de vitrales. Paraloid B-72 se ha usado com�nmente en la re-adhesi�n de pintura suelta. Sin embargo, a pesar de sus muchas ventajas (facilidad de uso, reversibilidad, buenas propiedades de envejecimiento, estabilidad y facilidad de mezclarse con diferentes tipos de solventes), han surgido algunas preguntas leg�timas con respecto a sus limitaciones (poca resistencia a la humedad, agarre relativamente pobre a substratos vidriosos y riesgos inherentes al solvente).Este art�culo comienza con una descripci�n de la qu�mica y caracter�sticas del Paraloid B-72 y su aplicaci�n en la conservaci�n de vidrios pintados hist�ricos. A continuaci�n se hace una revisi�n del estado actual de la investigaci�n del Paraloid B-72 y de las experiencias pr�cticas con el uso de este producto y TITULO—Revis�o de textos t�cnicos: uso de Paraloid B-72 como um consolidante de superf�cie para vidro colorido. RESUMO—Este artigo foi encomendado originalmente pelo English Heritage, que � �rg�o governamental consultor para arqueologia e ambiente hist�rico na Inglaterra, em meados da d�cada de 90 decorrente de questionamentos sobre a utiliza��o de Paraloid B-72 na consolida��o de pintura solta em tratamento de conserva��o de vidros coloridos. Paraloid B-72 tem sido utilizado habitualmente para a re-ades�o de pintura desprendida. Apesar das suas muitas vantagens (facilidade de uso, reversibilidade, durabilidade, estabilidade e miscibilidade em v�rios tipos de solventes), existem quest�es leg�timas sobre suas limita��es (baixa resist�ncia � umidade, liga relativamente fraca com substratos v�treos e perigos dos solventes).Este artigo come�a com uma descri��o da qu�mica e das caracter�sticas do Paraloid B-72 e sua aplica��o na conserva��o de objetos hist�ricos em vidro pintado. Segue-se uma revis�o da situa��o atual da pesquisa nesta �rea e experi�ncias pr�ticas no uso do Paraloid B-72 e sistemas correlatos. O artigo inclui um resumo das propriedades conhecidas e testadas do Paraloid B-72 em compara��o com materiais alternativos atualmente em estudo e usados na conserva��o de vidro colorido. Tamb�m discute os efeitos a longo prazo do tratamento e a necessidade de avalia��o constante de sua efic�cia. 1 INTRODUCTIONThis review looks at published sources of information regarding Paraloid B-72 and its role in consolidating stained glass, with particular reference to reattaching lifted or unstable paint layers. The aim is to comment on and evaluate recent trends in stained glass conservation practice. Paraloid B-72 has commonly been used to consolidate glass, either alone or in combination with other resins, since the early 1980s. In the museum environment, it is highly regarded as an adhesive for its strength, stability, and reversibility (Koob 1986; Horie 1987; Koob 2000). The ease with which the properties of the resin can be adapted to suit different circumstances by the addition of different types and quantities of solvent has made it a popular choice with conservators. It may, however, be more harshly tested in an architectural situation, where it may be subject to greater extremes of humidity, temperature, and UV radiation, and the resin may possess additional practical limitations as far as the treatment of stained glass is concerned. This study attempts to review and assess, based on sources available in the literature, the suitability of Paraloid B-72 for the treatment of architectural stained glass. The application of advanced scientific techniques to the study of the behavior of consolidants for different types of stained glass under artificial aging conditions has been largely a Franco-German endeavor. The last 10 years have seen a proliferation of literature on this subject and on the development of new materials specifically suited to the consolidation of glass (Romich et al. 1993; Programme Franco-allemand 1999; Wolff 2000), the salient features of which are reported here. The outcomes of these important and extensive studies—in particular the potential for development of new inorganic and hybrid consolidation products such as silicon-zirconium alkoxides (SZA) and ORMOCER—remain to be tested and observed in the practical domain. By contrast, empirical approaches to the treatment of objects on-site or in the laboratory, work-shop, or studio, practical experiences with consolidant products, and reviews of previous treatments are rarely reported in the literature of stained glass conservation. Wherever possible, provision should be made to disseminate this documentation more widely. This move would greatly assist researchers of the future, whose task it will be to evaluate past treatments, optimize existing systems, and enhance the properties of new products. 2 DESIRABLE CHARACTERISTICS OF A CONSOLIDANT FOR STAINED GLASSIn general terms, an appropriate consolidation treatment for stained glass may be required to improve the adhesion of the paint or painted layer to the glass substrate; improve cohesion of corroded layers of glass paint or stain; be able to cope with thermal and humidity conditions present at the site; possess excellent light-aging characteristics; be resistant to chemical attack; have minimal visual impact on the glass (i.e., not change the color, opacity, or refractive index of the painted layer); possess flow properties that enable it to penetrate the areas of loose paint (i.e., low viscosity and high surface tension); be chemically neutral, and remain so over time; be resistant to biodegradation; possess good application and handling characteristics; and be reversible (or at least not impede retreatment). In practice, the deterioration of paint on stained glass may involve different kinds of physical and chemical processes, depending on the way in which the stained glass is colored and fired. These processes can range from disaggregation of a fired surface layer and detachment from the glass substrate to lifting of flakes of applied paint. The consolidant must therefore be responsive to the particular nature of the glass in question and its degradation. This requirement makes it difficult to prescribe a single treatment that will work for all situations. 2.1 THE CONSOLIDANTSPolymers used as consolidants can be divided into two main groups: natural and synthetic. Some natural polymers are relatively stable and have been used in the past for glass repair (Davison 1984). There has been some recent interest in and comparative studies of the potential of natural wax mixtures (beeswax, carnauba wax) for the consolidation of glass paint, and the suitability of these is being monitored in situ (M�ller 1984; Forberg and Bornschein 1996). However, synthetic polymers are generally preferred in conservation on account of the large body of data, acquired over many decades of experimental and laboratory research, that support their stability and other favorable properties. Some synthetics, notably the acrylics, show good aging characteristics. Other synthetic resins—for example, soluble nylon and polyvinyl acetate—are unsuitable, as they can attract dust, which is an undesirable characteristic in a consolidant. In the case of polyvinyl acetate, which has a low glass transition point (Tg) of 28�C, there is also a tendency for the material to cold flow (Newton and Davison 1989).1 For the consolidation of historic stained glass, two broad types of synthetic resin system have been chiefly reported in the literature: epoxies and acrylics. Other materials, such as polyurethanes, have also been used experimentally. 2.1.1 EpoxiesFollowing early concerns about yellowing and degradation of the resin over time, tests reported the availability of epoxy resin systems that possess acceptable lightfastness and clarity (Tennent 1979; Down 1986). A number of epoxies have been successfully employed, chiefly as adhesives and gap fillers for broken glass fragments (Notman and Tennent 1980; Jackson 1982; Davison 1984; De Henau and Fontaine-Hodiamont 1991). Less successful has been the use of epoxy resins dissolved in solvents such as acetone, or low-viscosity room-temperature–curing epoxies such as Ablebond 342-1, as consolidants for painted glass and grisaille (Weintraub and Greenland 1984; Cole 1989; Femenella 1994; Strobl 1999). 2.1.2 AcrylicsThe monomers from which acrylic polymers are made fall into two groups: acrylates and methacrylates. Polymethacrylates were among the first synthetic resins used to coat glass (Bettembourg 1976; Newton and Seddon 1999) and as consolidants are still among the most popular. The Paraloid (formerly Acryloid in the U.S.) resins, discussed more fully below, are perhaps the best known. 2.1.3 Other MaterialsA third class of materials, polymers formed by reaction of a polyisocyanate with a polyacrylate, and sometimes categorized in the literature as polyurethanes, has been used experimentally. Systems based on the acrylate resin Viacryl SM564 + Desmodur N75 (an isocyanate) were developed and tested during the 1970s (Bettembourg 1976; Newton and Seddon 1999), but these particular resins were shown to be unsuitable for the situations in which they were used and are no longer employed in stained glass conservation. However, these kinds of polymer remain potentially useful materials for the protective coating of glass (Newton 1987). Recent research on consolidation of stained glass has also shown that two-part acrylate-isocyanate systems (Desmophen A160–Desmodur N75) perform very well in terms of adhesion, optical stability, and durability when compared to other materials, including methacrylates (J�gers et al. 2000). These systems are, however, irreversible. Limited application has also been found for ethyl silicates, which are theoretically interesting as they enrich the glass with silica, but are also irreversible (Fontaine 1999). 2.2 PARALOID (ACRYLOID) RESINSThere are several types of Paraloid available, including B-44, B-66, B-67, and B-72. They are all thermoplastic acrylic resins available as solution grade or as solid grade acrylic resin (Table 1). 2.2.1 Paraloid B-44Paraloid B-44 is used in conservation and is slightly harder than B-67. It has a Tg of 60�C and is a methyl methacrylate copolymer (MMA). The manufacturers say that in its solute form, Paraloid B-44 is often blended with other Paraloid resins to adjust those resins to the balance of properties required for a particular application. 2.2.2 Paraloid B-66Paraloid B-66 is a general-purpose resin with very fast solvent release. It is somewhat softer than B-44 but harder than B-72. 2.2.3 Paraloid B-67Paraloid B-67 is the most water-resistant of Paraloid resins. It provides a coating that is hard and fast drying and imparts good gloss and color retention. It has a Tg of 50�C and is an i-butyl methacrylate polymer (IBMA). It has been used as a picture varnish and is soluble in less polar solvents2 (such as white spirit) than B-72. 2.2.4 Paraloid B-72Paraloid B-72 is a copolymer of ethyl methacrylate and methyl acrylate (70: 30) manufactured by Rohm and Haas. Paraloid B-72 is a very stable resin with a Tg of 40�C and a refractive index3 of 1.49. The popularity of Paraloid B-72 owes as much to its versatility as to its apparent stability. It is sold either in solution or in solid pellet form. The pellets can be dissolved with a compatible solvent to the desired concentration, so that the conservator can vary the viscosity of the resin to suit the application. As mentioned above, Paraloid B-72 is an ethyl methacrylate: methyl acrylate P(EMA-MA) with a molar ratio of 70: 30. Some concern was expressed about the use of Paraloid B-72 in conservation after the manufacturer changed its composition in 1978. The resin originally had a molar ratio of 68: 32 ethyl methacrylate: methyl acrylate P(EMA-MA) with a lower molecular weight and was soluble in slightly less polar solvents. De Witte and coworkers (1978) carried out a variety of tests to confirm the difference in chemical makeup and concluded that the change would probably not affect the stability of the resin but that long-term aging tests should be considered. 2.3 LIMITATIONS OF PARALOID RESINS FOR STAINED GLASS CONSOLIDATIONThe inherently weak chemical bond between the organic consolidants generally used in conservation and the inorganic and weathered gel layer has been
The chief limitation in terms of durability of acrylic resins such as Paraloid B-72, on the other hand, has to do with their poor water resistance and the problem of permeability in plastics. The degree to which Paraloid resins are susceptible to these phenomena depends to some extent on the form and concentration in which the resin is applied. Accordingly, authors have been rightly circumspect about the wisdom of using acrylic resins in situations of unbuffered exposure to weathering agents (Sloan 1995). But these limitations have not inhibited the widespread use of Paraloid B-72 in cases in which objects or treated surfaces are not subject to high humidities (and to a lesser extent temperatures). In the case of ancient stained glass in an architectural setting, it is common practice to install protective glazing. Removal of the impact of exterior weathering factors and stabilization of internal temperature and humidity conditions can create an environment that is better suited to preventive conservation of glass paint. Where protective glazing is to be installed, tests and field studies have reported on the suitability of Paraloid B-72 in toluene or another solvent for the consolidation of glass paint and grisaille (Tr�mpler et al. 1996). Nonetheless, accurate microclimatic evaluations are required to assess the likely impact of temperature and humidity ranges on proposed conservation-restoration products, and the properties of a given resin formula must be known. The question of permeability concerns the risk of water vapor's penetrating a resin film, where over time salts or pollutant compounds trapped beneath may attack the glass substrate. The difficulties involved in creating an effective moisture barrier in the surface treatment of ancient glass using organic resin coatings have been appreciated for many years (Newton 1974; Moncrieff 1975). Both resin concentration and solvent type can significantly influence Additional practical limitations, also concerning resin concentration and solvent type, do give cause for concern in the context of architectural stained glass. By mixing Paraloid B-72 with a solvent at concentrations typically between 2.5% and 7.5%, a low-viscosity material capable of penetrating corroded glass surfaces can be manufactured easily. An experienced conservator can modify the characteristics of the resin to ensure the best result: too low a concentration, and the resin lacks adhesive strength and can be difficult to control; too high, and penetration of the porous glass matrix may be inadequate, while darkening of the surface may be more pronounced. The solvents most commonly used in association with Paraloid B-72 are acetone, toluene, xylene, ethyl acetate, and diacetone alcohol. The correct choice of solvent and the solvent-solid ratio are important factors in obtaining a resin with suitable workability and flow characteristics. For stained glass this choice must be based, among other things, on the type and condition of the glass paint. For Paraloid B-72 as an adhesive, Koob (1986) has outlined the advantages of acetone, which in addition to being less toxic than some of the others, is released more quickly. Acetone has often been selected as the solvent in the case of stained glass treatments (Learner and Bettembourg 1991), although for consolidation of corroded pigment layers or poorly fired glass paint, some conservators have found that acetone evaporates too quickly. Penetration capacity is reduced, and the ratio of solvent to resin is more difficult to control (Strobl 1999).4 Hence the less volatile solvents have been considered more suitable for use as a consolidant for stained glass, toluene often giving good results. The experience of Tr�mpler and coauthors (1996) indicates that where a grisaille surface is relatively porous, use of toluene has the advantage of securing loose particles relatively quickly and permitting the removal of excess resin before curing takes place. Similarly, Mueller-Weinitschke (1996) reports on the superior properties of toluene over the more volatile alternatives. Health and safety risks of some of these solvents are well understood. But the handling and application difficulties that these authors comment on may have a direct impact on the effectiveness of the resin consolidant. Most important, because Paraloid resins cure by solvent evaporation, reliable consolidation depends on the ability of the resin solution to penetrate the weakened glass matrix effectively before the resin cures. De Henau (1996) has noted that where Paraloid B-72 is used with a pure solvent, the evaporation rates of the solvent may be too high to ensure adequate consolidation in the depth of a porous body. The faster the solvent evaporates, the more rapidly its viscosity increases and the greater the risk of inadequate penetration of the resin through the porous material. Choosing the solvent most suited to the particular case in question is thus no less important than the choice of resin. Even the use of a slower-evaporating solvent is not without potential risks. Slow solvent release can mean longer setting times and solvent retention after the initial cure. For refixing of enamel, De Henau (1996) has carried out controlled tests using 10% B-72 in p-xylene and demonstrated how such shortcomings manifest themselves where the evaporation surface of the consolidant is small in relation to the volume of resin applied. For some types of loose or corroded glass paint, repeated application of the consolidant is sometimes necessary. Some authors have considered that for badly corroded and granular glass paint with very poor cohesive strength, the use of acrylics such as Paraloid B-72 is inappropriate. The increase in gloss and darkening of the surface resulting from the multiple applications needed to secure areas of severe decay are unacceptable (Weintraub and Greenland 1984). Using simulated grisaille panels, Mueller-Weinitschke (1996) has demonstrated the effect, showing that multiple applications of Paraloid B-72 in toluene at 5–7.5% are needed in order to adequately secure the less well-fired areas of grisaille. The degree of darkening is a function of the percentage concentration of resin and the number of applications required. The light-and heat-aging properties of acrylates have been extensively studied, especially in relation to It is clear, therefore, that the very adaptability of Paraloid resins imposes a greater need for careful scrutiny of the variation of effects observed in different conditions, and on different substrates, using different resin concentrations, solvent types, and methods and numbers of applications. 3 NEW MATERIALSThere has been much focus in recent years on the potential application in the stained glass field of two newly developed materials: ORMOCER (heteropoly-siloxane compounds) and silicon-zirconium alkoxides (SZA). ORMOCER, developed originally as protective coatings, have been found to have some potential application in hybrid treatments, though they adhere poorly to glass (Mueller-Weinitschke 1996) and are therefore not suitable as consolidants. SZA, however, appeared to offer some promise during early tests (Romich et al. 1995). Though not reversible, SZA treatment, which relies on sol-gel chemistry rather than mechanical adhesion to bind together friable particles, is chemically more suited to the consolidation of silicate materials than the “film-forming” synthetics. After application, the silicon and zirconium alkoxides react with moisture in the air to form an inorganic gel. SZA appears to have no impact on the optical properties of treated glasses, and it has good penetration properties. Practical experience of SZA, however, has been discouraging (Mueller-Weinitschke 1996). It has been found to be very sensitive to humidity, not hydrolyzing fully unless relative humidities exceed 50%, and difficult to control owing to its very low viscosity. It is less effective as an adhesive, so loose flakes or detached parti-cles—i.e., those areas of a corroded glass surface that are most at risk—cannot be effectively re-adhered. In short, SZA appears to offer some potential but lacks the ease of handling and versatility of B-72. Further comparative studies are being undertaken, and observations on the field trials, begun in 1990, are awaited. 3.1 OPTIMIZATION OF PARALOID-BASED TREATMENTSExamples in the literature report some of the recent attempts to refine one or more of the properties of Paraloid-based consolidants. They typically fall into three categories:
3.1.1 Silane Coupling AgentsThe adhesive bond of polymers to glasses can be improved by the use of coupling agents. The performance of silane coupling agents in several studies in the 1980s indicated the conservation benefits that may be derived from their use in combination with Paraloid resins for the consolidation of loose paint. Errett et al. (1984) described the process of silane pretreatment and, to examine the effectiveness of the silane in promoting wetting and spreading, treated a number of slides with a methacrylate functional silane in methanol, then applied a solution of B-72 on the following day. On average, the consolidant flowed twice as far in the silane-treated interface as in the untreated interface. Reviewing the approach to the treatment of stone using similar methods, Jones (1988) adapted the technique for the treatment of remaining unstable 3.1.2 ORMOCERSLike silanes, ORMOCER, which as heteropolysiloxane compounds are well-suited to bonding chemically with silicate surfaces, offer significant potential as additives to Paraloid resin consolidants. Tr�mpler and coworkers (1996) are among those who have found a 50: 50 mixture of ORMOCER Or-G and 10% Paraloid B-72 in ethyl acetate to possess good characteristics in terms of both handling and penetration power. The ORMOCER-Paraloid combination is also being tested for the treatment of flaking enamel (M�ller et al. 2000). Pilz (1999), meanwhile, has reported on the positive outcome of laboratory tests on simulated medieval glasses in which the combination of SZA as consolidant and 50: 50 ORMOCER-Paraloid B-72 as a protective film, applied after the glass samples have been subjected to hypercritical drying, have been deployed on glass darkened as a result of manganese oxidation. The transfer of the technique to the studio, however, remains problematic. 3.1.3 Solvent ChemistryThe third approach, which seeks to improve the wetting properties and penetration of the solution and also to control more finely the evaporation rate of the solvent, has yielded interesting results on some types of glass (De Henau 1996). Fontaine has described the treatment of corroded archaeological glass using 10% Paraloid B-72 in a 3: 2 solvent: diluent mixture of methanol and ether (Fontaine-Hodiamont 1993). 4 CONCLUSIONSIt is widely accepted that minimum intervention is desirable in the treatment of any object, and where uncertainties exist about the long-term suitability of treatments, this maxim is especially important. The duty of the conservator is to consolidate loose and friable surface layers only where there is a risk of loss of valuable historic material and to employ any consolidating technique with restraint and sensitivity. Increasingly, the value of preventive conservation is becoming evident, and protective glazing systems will continue to play a primary role in the preservation of historic glass paint. However, consolidation of glass paint remains a necessary and important undertaking, and the choice of consolidant system is one of the most important a conservator can make. The chemical composition of the glass, glass paint, and their corrosion products, the type and degree of alteration of the surface layers, the presence of earlier coatings or treatments, the nature of any associated interventions (re-assembly, crack repair, cleaning, surface treatment), the environmental conditions to which the treated panel will be subjected—all play a part in determining which, if any, are the most suitable consolidants. To date, Paraloid B-72 has shown itself to be versatile and effective as well as stable under moderate conditions. There are certain practical limitations to its use as a consolidant, notably the nature of the adhesive bond with inorganic materials and the ability of the resin and its carrier to both penetrate and consolidate effectively the fine pores and fissures of a silicate matrix. A review of the literature illustrates some of the ways in which Paraloid B-72 can be optimized to reduce some of the inherent shortcomings. Paraloid B-72 possesses two singular advantages over its chief rivals: first, it has been around for many decades and has shown itself to be reliable if used in an appropriate context. Second, it is readily reversible and miscible with a wide range of solvents and can be easily combined with other materials to enhance its properties. Reversibility, which may be crucial in an adhesive, can be an overestimated virtue in a consolidant, where the removal of the consolidant would entail The literature indicates that systematic reviews of the long-term performance of Paraloid-treated glass surfaces in the field are rare. While laboratory aging studies continue, these must be corroborated by surveys of treated panels in situ. More data are also needed from pilot studies on panels treated with alternative materials and on modified Paraloid-based systems. Techniques and methodologies for this research should be developed to enable a more comprehensive picture of the comparative performance of various treatments to be built up. NOTES1. Tg or glass transition temperature is measured as that temperature at which the available thermal energy is smaller than the forces holding molecules together. At lower temperatures, very little molecular adjustment is possible. Below its Tg, an amorphous polymer is brittle and hard; above its Tg, it is softer and can be dissolved more easily. 2. Polar is descriptive of a molecule in which the positive and negative electrical charges are permanently separated, as opposed to nonpolar molecules in which the charges coincide. Polar molecules ionize in solution and impart electrical conductivity. Water, alcohol, and sulfuric acid are polar in nature; most hydrocarbon liquids are not. Carboxyl and hydroxl groups often exhibit an electric charge. The formation of emulsions and the action of detergents are dependent on this behavior. 3. Refraction is the change in direction (apparent bending) of a light ray passing from one medium to another of different density, as from air to water or glass. The ratio of the sine of the angle of incidence to the sine of the angle of refraction is the index of refraction of the second medium. Index of refraction of a substance may also be expressed as a ratio of the velocity of light in a vacuum to its velocity in the substance. It varies with the wave length of the incident light, temperature, and pressure. The usual light source is the D line of sodium, the standard temperature being 20�C. The expression of refractive index is nD20. 4. Horie (1987) describes the chemistry of p-xylene, which evaporates more slowly during the initial wet stage of drying but is released freely during the later stages. This characteristic is ascribed to the difference in polarity in the solvents. REFERENCESBettembourg, J.-M.1976. Protection des verres de vitraux contre les agents atmosph�riques: Etude des films de r�sines synth�tiques. In Verre et r�fractaires. Actes du IX colloque international du Corpus Vitrearum Medii Aevi, Paris, 1975. 87–91. Cole, F.1989. Stained glass conservation at Canterbury Cathedral. In Conservation of stained glass. United Kingdom Institute for Conservation Occasional Papers 9. London: UKIC. 3–4. Davison, S.1984. A review of adhesives and consolidants used on glass antiquities. In Adhesives and consolidants, ed. N. S.Brommelle et al. London: International Institute for the Conservation of Historic and Artistic Works. 191–94. De Henau, P.1996. Le Paraloid B-72 pour fixer la grisaille ou l'�mail? In Grisaille, jaune d'argent, sanguine, �mail et peinture � froid: techniques et conservation. Forum pour la conservation des vitraux, Li�ge. Li�ge: Commission Royal des Monuments, Sites et Fouilles. 139–42. De Henau, P., and C.Fontaine-Hodiamont. 1991. Aspects de la conservation de vitraux en Belgique. In Les arts du verre: Histoire, technique et conservation. Journ�es d'�tude de la SFIIC (Section Francaise of the International Institute for Conservation). Nice: SFIIC. 59–63.
De Witte, E., M.Goessens-Landrie, E. J.Goethals, and Down, J.1986. The yellowing of epoxy resin adhesives: Report on high intensity light aging. Studies in Conservation31: 159–70. Errett, R. F. M.Lynn, and R.Brill. 1984. The use of silanes in glass conservation. In Adhesives and consolidants, ed. N. S.Brommelle et al. London: International Institute for the Conservation of Historic and Artistic Works. 185–90. Femenella, A. J.1994. Restoring stained glass paint. Stained Glass89(1):43–48. Fontaine, C.1999. Conservation of glass at the Institut Royal du Patrimoine Artistique (Brussels): From the earthquake in Li�ge to the stained glass of Loppem. In Conservation of glass and ceramics: Research practice and training, ed. N. H.Tennent. London: James & James. 199–207. Fontaine-Hodiamont, C.1993. Quatre-vingt neuf verres tres fragmentaires provenant du site de Khirbet Qumr�n (Cisjordanie, Ier si�cle ap. J-C). Bulletin de l'Institut Royale du Patrimoine Artistique25: 277–80. Forberg, H.-D., and Bornschein, F.1996. Vitraux m�di�vaux de la Cath�drale d'Erfurt: D�t�riorations et solutions pour la conservation du patrimoine. In Grisaille, jaune d'argent, sanguine, �mail et peinture � froid: Techniques et conservation. Forum pour la conservation des vitraux, Li�ge. Li�ge: Commission Royal des Monuments, Sites et Fouilles. 169–74. Horie, C. V.1987. Materials for conservation.London: Butterworths. Jackson, P. R.1982. Resins used in glass conservation. In Resins in conservation, ed. J. O.Tate et al. Edinburgh: Scottish Society for Conservation and Restoration, 10. 1–7. J�gers, E., H.R�mich, and C.Mueller-Weinitschke. 2000. Konservierungsmaterialen und Methoden. In Restaurierung un Konservierung historischer Glasmalerein: Ein F�rderprojekt des Budesministeriums f�r Bildung, Wissenschaft, Forschung und Technologie, ed. A.Wolff. Mainz: Verlag Philipp Von Zabern. 129–66. Jones. S.1988. The conservation of three fourteenth century stained glass lancets. In Conservation today: Papers presented at the United Kingdom Institute for Conservation. London: UKIC. 86–97. Koob, S. P.1986. The use of Paraloid B-72 as an adhesive: Its application for archaeological ceramics and other materials. Studies in Conservation31:7–14. Koob, S. P.2000. New techniques for the repair and restoration of ancient glass. In Tradition and innovation, ed. A.Roy and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 92–95. Learner, E. and J.-M.Bettembourg. 1991. Restauration et conservation des vitraux fran�ais de Wilton (GB). Vitrea7:43–53. Moncrieff, A.1975. Problems and potentialities in the conservation of vitreous materials. In Conservation in archaeology and the applied arts. London: International Institute for Conservation of Historic and Artistic Works. 99–104. Mueller-Weinitschke, C.1996. Experiences de consolidation des traits de contour: Une �tude comparative de plusieurs substances utilis�es pour la fixation des traits de contour. In Grisaille, jaune d'argent, sanguine, �mail et peinture � froid: Techniques et conservation. Forum pour la conservation des vitraux, Li�ge. Li�ge: Commission Royal des Monuments, Sites et Fouilles. 85–89. M�ller, W.1984. Untersuchungen zur Schutzwirkung organischer Beschichtungen auf simulierten mittelalterlichen Gl�sern. Neue Museumkunde27(4):273–75.
M�ller, W.1996. Le probleme de la fixation du M�ller, W., D.Kruschke, C.Kocher, M.Pilz, H. R�mich, and C.Troll. 2000. Welches Festigungsmittel eignet sich? Experimentelle Forschungen an der BAM und am ISC. Restauro106(6):442–46. Newton, R.1974. The deterioration and conservation of painted glass: A critical bibliography and three research papers. Corpus Vitrearum Medii Aevi, Great Britain Occasional Papers 1.London: Oxford University Press. Newton, R.1987. What do we really know about protective coatings? ICOM Committee for Conservation preprints, 8th Triennial Meeting, Sydney. Los Angeles: ICOM. 1009–12. Newton, R., and S.Davison. 1989. Conservation of glass.London: Butterworths. Newton, R., and A. B.Seddon. 1999. Organic coatings for medieval glass. In Conservation of glass and ceramics: Research practice and training, ed. N. H.Tennent. London: James & James. 66–71. Notman, J. H., and N. H.Tennent. 1980. The conservation and restoration of a seventeenth century stained glass roundel. Studies in Conservation25:165–75. Pilz, M.1999. Traitements de conservation pour les vitraux alteres et brunis. In Monuments historiques et environnement: Recherches franco-allemands sur la conservation de la pierre et du vitrail 1988–1996/Baudenkmaler und Umwelt: Deutsch-Franz�sische Forschungen zur Erhaltung von Natursteinen un Glasmalereien 1988–1996. Programme Franco-allemand de recherche pour la conservation des monuments historiques, colloque final, Strasbourg 1997. Paris: Exe Productions. 330–46. Programme Franco-allemand/Deutsch-Franz�sisches Forschungsprogramm. 1999. Monuments historiques et environnement: Recherches franco-allemands sur la conservation de la pierre et du vitrail 1988–1996/Baudenkmaler und Umwelt: Deutsch-Franz�sische Forschungen zur Erhaltung von Natursteinen un Glasmalereien 1988–1996. Programme Franco-allemand de recherche pour la conservation des monuments historiques, colloque final, Strasbourg 1997. Paris: Exe Productions. Romich, H., M.Pilz, and D. R.Fuchs. 1993. Konservierung Historischer Glasfenster. Internationale Untersuchungen neue Methoden. Forschungsbericht Teil 2. Research report on the Umweltforschungsplan (UFOPLAN) n. 10807 005/03 of the German Federal Ministry of the Environment. Berlin: Umweltbundesamt. Romich, H., M.Pilz, and D. R.Fuchs. 1995. A new material for glass conservation: Perspectives for ceramics heritage. In The ceramics heritage, ed. P.Vincenzini. Proceedings of the CIMTEC 8th World Ceramics Congress and Forum on New Materials, Florence, Italy. Faenza, Italy: Techna. 613–20. Sloan, J.1995. The restoration of stained glass windows at Memorial Hall, Harvard University: A case study. APT Bulletin (Association for Preservation Technology)26:2–3. Strobl, S.1999. Paint consolidation at Canterbury Cathedral. CVMA Newsletter (Corpus Vitrearum Medii Aevi) 46: 37. Tennent, N. H.1979. Clear and pigmented epoxy resins for stained glass conservation: Light ageing studies. Studies in Conservation24:153–64.
Tr�mpler, S., J.James, and S.Gentile. 1996. Le traitement des grisailles alt�r�es. In Cath�drale de Lausanne: Colloque du 31 Mai 1996. Restauration des verri�res m�di�vales de la Rose: Documentation preparatoire. Lausanne: �tat de Vaud Departement des Travaux Publics, de l'Am�nagement et des Transports. 23–28. Weintraub, S., and M.Greenland. 1984. Field application of stained glass conservation techniques. In Adhesives and consolidants, ed. N. S.Brommelle et al. London: International Institute for the Conservation of Historic and Artistic Works. 199–201. Wolff, A., ed.2000. Restaurierung un Konservierung historischer Glasmalerein: Ein F�rderprojekt des Budesministeriums f�r Bildung, Wissenschaft, Forschung und Technologie. Mainz: Verlag Philipp Von Zabern. AUTHOR INFORMATIONSASHA CHAPMAN originally trained as an archaeologist specializing in recording of buildings. She joined English Heritage (the government's statutory advisor on archaeology and the historic enviornment in England) in 1993 and was involved in coordinating research and technical advice in several areas, particularly graffiti removal. She is a former chair of the United Kingdom Institute for Conservation of Historic and Artistic Works, Stone and Wall Paintings Section. DAVID MASON trained in fine art, worked as a stone conservator, and gained a Ph.D. from De Montfort University in Leicester, England, where he specialized in the history and theory of conservation. He joined the Building Conservation and Research Team at English Heritage in 1997. He has coordinated BCRT technical research and was editor of English Heritage Research Transactions from 1999 to 2001.
 Section Index Section Index |