ADHESIVE REPLACEMENT: POTENTIAL NEW TREATMENT FOR STABILIZATION OF ARCHAEOLOGICAL CERAMICSMICHAELA NEIRO
2 TREATMENTThe customary method for treating ceramics bonded with an unstable adhesive involves preparation of a map of the reconstruction, complete disassembly of the vessel, painstaking removal of adhesive from each shard, and reconstruction of the vessel with a more stable adhesive. Depending on the size of the object and the complexity of the breaks, this process can take from 8 to 40 hours or more. When considering the magnitude of archaeological ceramics in many museums, this task seems unduly burdensome and expensive. The strategy behind the adhesive replacement method is for the object to remain whole during treatment. Using this method, the majority of the cellulose nitrate is systematically wicked out of the join interfaces with acetone and replaced with Paraloid B-72 (formerly Acryloid—ethylmethacrylate copolymer). Only one section of the vessel is worked on at a time, allowing the whole object to remain intact throughout the treatment. Cellulose nitrate is readily soluble in acetone. This solvent can be obtained easily and inexpensively and is among the least toxic of commonly used solvents. Paraloid B-72 was chosen as the adhesive for this technique due to its reputation as a good adhesive for ceramics, its ease of use, and its long-term stability. Paraloid B-72 is not as hard or as brittle as cellulose nitrate and therefore is better able to tolerate the stresses on the ceramic joins (Koob 1986). Greg Byrne, objects conservator at the National Park Service laboratory in Harpers Ferry, West Virginia, first performed the adhesive replacement treatment in 1986. The object treated was an eastern Native American porous, coarse, unglazed earthenware pot from Colonial National Historical Park. The method used in 1986 was fairly similar to the method illustrated in this article, although polyvinyl acetate (PVAC) AYAF (a high-viscosity, 80 centipoise, thermoplastic resin with a Tg of 24�C) was the adhesive chosen for replacement. Twelve years later, in 1998, the vessel was sent to a private conservation laboratory for examination to determine its stability with regard to potential travel. The object was examined visually through a stereo microscope and physically
Six objects from Colonial National Historical Park, Jamestown, Virginia, were used to test this method (table 1). The stability of the ceramics before treatment was measured—visually by noting gaps, fills, losses, and flaking surfaces, and audibly by lightly tapping a vessel in several areas and noting the tonal clarity or lack thereof. The reconstructed objects consisted of approximately 15–30 shards with some voids and plaster fills present. With the exception of a single pearlware object, all were earthenware vessels of medium porosity with a light brown/orange clay body. The European pearlware vessel was thinner with a less porous, white body. Overall, the old adhesive had been applied very carelessly, with wide bands extending over the break edges and drips scattered throughout (fig. 2). The vessels were examined and photographed before and after treatment under ultraviolet illumination. Because cellulose nitrate fluoresces bright yellow under ultraviolet illumination, it was easy to see the adhesive within and surrounding all the joins. 2.1 PREPARATIONIn preparation for treatment, the practitioner should have a table set up with fume extraction, a method for supporting the object, two bottles of acetone labeled “clean” and “dirty,” several brushes, cotton, paper towels, a flashlight, a bottle of distilled water, and 30% Paraloid B-72 in ethanol: acetone (3:1). Acetone alone is used to dissolve the CN; it works fast, evaporates quickly, and is less likely to drive the CN into the clay body. Ethanol: acetone (3:1) is the solvent system for Paraloid B-72. The acetone solubilizes the Paraloid B-72 and the ethanol
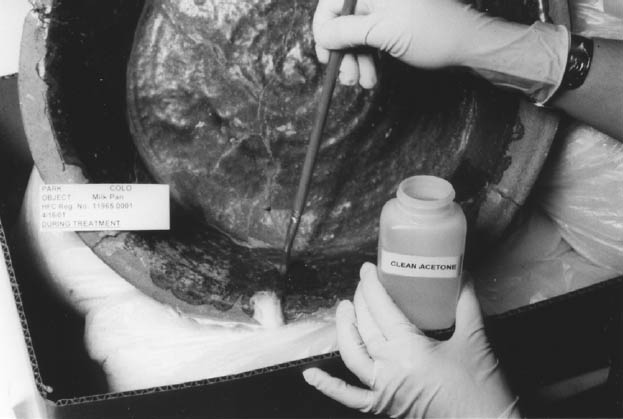
Distilled water was liberally applied by brush to the ceramic on each side of the join to be treated. If salts are present in the ceramic, an alternative nonaqueous solvent would be more appropriate than water for this purpose. Pretreating the site with water filled the porous ceramic and thereby limited the intrusion of the solubilized adhesive solution during solvent treatment (Buys and Oakley 1993). This water barrier method proved effective in preventing tidelines around the breaks on the objects in this study. Having glaze present on one or both sides of the ceramic naturally reduces the possibility of tidelines. Next, a wad of long-fiber cotton was stretched into a strip approximately 1.0 cm wide and placed on the exterior of the vessel along the length of the first join to be treated. The purpose of the cotton was to absorb flushed-out solvent and old, yellow adhesive. The cotton wads were systematically replaced when they became saturated, until the join was free of adhesive. 2.2 REMOVAL OF CELLULOSE NITRATEIt is best to begin with the tightest joins first. If significant gaps exist between the shards, they can be minimized toward the end of the process, while the adhesive is slightly soft. Each object is a puzzle, and with practice, one sees which pieces are more or less crucial in holding the vessel together. Not concentrating in one area proved most effective in moving quickly around the object while keeping it in one piece. With a soft, medium-sized brush, acetone is liberally applied along the inside of the selected break edge (pretreated with water from the previous step), flushing the join with solvent. Old cellulose nitrate is easily soluble in acetone, and the adhesive is quickly wicked into the cotton wadding in place on the other side of the join and is also lifted away with the brush. The brush is wiped clean on a paper towel and rinsed in a separate bottle of acetone labeled “dirty.” With the acetone from the “clean” bottle, the brush is used again to apply acetone to the cellulose nitrate–filled join and to lift out excess adhesive. A brush was found to be more useful than a pipette or a syringe, as it not only introduced the solvent but also lifted out the old adhesive. A significant amount of adhesive can be removed by the brush, therefore limiting the amount of solubilized adhesive that passes into the ceramic. Continually flushing the join with acetone quickly saturated the cotton wadding. The cotton released easily from the vessel in a tidy, soaked strip and was replaced until the acetone flushed clear and no trace of yellow adhesive was wicked into the cotton. Cotton linters stuck to the ceramic were not a problem due to the substantial amount of acetone present. When very tight joins needing treatment were encountered, the same method was employed. However, it was more difficult to determine at what point the old adhesive has been flushed from the join. Both a flashlight and a fiber optics light source were useful tools in determining when the join was free of adhesive (fig. 3). At this stage there is the opportunity to clean up any excess cellulose nitrate that may be found on the surface of the vessel. The adhesive replacement treatment is not meant for cosmetic purposes, but, when appropriate, this can be an important step and does not take much extra time. A skin of brittle adhesive on a glazed surface is likely to flake, often taking the glaze with it, resulting in significant damage and loss of information. Several of the objects in this study had sloppily applied adhesive on their glazed surfaces that was brittle and was causing the glaze to flake. Using the brush and acetone, the adhesive can be removed or reduced, and the glaze flake can be saved from detachment. The cellulose nitrate can be reduced and manipulated to tack down the glaze flakes until Paraloid B-72 can be wicked in during the following treatment stage.
One of the objects treated contained an old plaster fill. This fill was stable and firmly attached to the shards adjoining it, so it was not removed. Again, it must be stated that this treatment is purely for stabilization purposes. Gaps or voids may be present between shards after treatment, but as long as there is good contact between most of the surfaces, the shards will remain in place. 2.3 ADDITION OF B-72When the acetone and the brush remain clean and there is no visual trace of old adhesive, a generous amount of Paraloid B-72 (30% by weight) in ethanol |