ADHESIVE REPLACEMENT: POTENTIAL NEW TREATMENT FOR STABILIZATION OF ARCHAEOLOGICAL CERAMICSMICHAELA NEIRO
ABSTRACT—The treatment described here was developed to address the problem of masses of deteriorating archaeological ceramics in need of reconstruction in a cost-effective manner. Archaeological ceramics form a major portion of many museum collections, including those of the National Park Service. Cellulose nitrate adhesives were the material of choice for the reconstruction of ceramics by archaeologists from the 1930s through the late 1970s and are still being used to some extent today. This adhesive is now failing in many old repairs. Greg Byrne, objects conservator at the National Park Service laboratory in Harpers Ferry, West Virginia, contrived a novel method for the re-treatment of archaeological ceramics by adhesive replacement. As part of my third-year internship at Harpers Ferry Center, I explored this technique further. TITRE—La substitution in situ d'un adh�sif par un autre: un nouveau traitement potentiel pour stabiliser les c�ramiques arch�ologiques. R�SUM�—Le traitement d�crit dans cet article fut d�velopp� comme solution �conomique au probl�me pr�sent� lors du re-traitement d'une �norme quantit� de c�ramiques arch�ologiques en train de se d�t�riorer. Les c�ramiques arch�ologiques sont une composante importante de plusieurs collections mus�ales, dont la collection du National Park Service (service national des parcs). � partir des ann�es 1930 jusqu'� vers la fin des ann�es 1970, et m�me dans certains cas jusqu'� ce jour, les adh�sifs de pr�dilection des arch�ologues pour le remontage des c�ramiques �taient ceux � base de nitrate de cellulose. Ce type d'adh�sif s'est maintenant d�t�rior� et risque de c�der dans de nombreux exemples d'anciens traitements. Greg Byrne, restaurateur d'objets au laboratoire du service national des parcs � Harpers Ferry, en Virginie de l'Ouest, a con�u une nouvelle m�thode pour retraiter ces c�ramiques, fond�e sur la substitution in situ de l'adh�sif original par un nouvel adh�sif plus stable. L'auteur de cet article a poursuivi le d�veloppement de cette technique lors de son stage de troisi�me ann�e d'�tudes � ce m�me laboratoire (Harpers Ferry Center). TITULO—Reemplazo del adhesivo: un posible nuevo tratamiento para la estabilizacion de ceramica arqueologica. RESUMEN—El tratamiento descrito aqu� fue desarrollado como soluci�n econ�mica al problema de la gran cantidad de cer�mica arqueol�gica deteriorada que necesita reconstrucci�n. La cer�mica arqueol�gica constituye una porci�n importante de las colecciones de muchos museos, incluidos los del National Park Service (NPS) (Servicio nacional de parques). Los adhesivos a partir de nitrato de celulosa fueron el material elegido para la reconstrucci�n de cer�mica por los arque�logos desde los a�os 30 hasta el fin de los a�os 70 y todav�a se usan hoy en alguna medida. Este adhesivo est� fallando ahora en muchas reparaciones anteriores. Greg Byrne, el conservador de objetos del laboratorio del NPS en Harpers Ferry, West Virginia, ide� un m�todo nuevo para tratar de nuevo la cer�mica arqueol�gica por medio del reemplazo del adhesivo. Como parte del tercer a�o de mi pasant�a en Harpers Ferry Center, continu� la exploraci�n de esta t�cnica. TITULO—Substitui��o de adesivo: novo tratamento para estabiliza��o de cer�mica arqueol�gica. RESUMO—O tratamento aqui descrito foi desenvolvido para abordar o problema de deteriora��o em massa de cer�mica arqueol�gica que necessita de reconstru��o, levando em conta o custo-benef�cio. Cer�micas arqueol�gicas formam a maior parte das cole��es de muitos museus, inclusive as do National Park Service (Servi�o Nacional de Parques). Dos anos 30 at� o final dos anos 70, os adesivos de nitrato de celulose eram o material escolhido pelos arque�logos para a reconstru��o de cer�mica e ainda hoje s�o utilizados at� certo ponto. Atualmente este adesivo est� enfraquecido em muitos reparos antigos. Greg Byrne, restaurador de objetos nos laborat�rios do National Park Services em Harpers Ferry, West Virginia, inventou um novo m�todo para tratar novamente cer�mica arqueol�gica por substitui��o de adesivo. Aprofundei esta t�cnica em meu est�gio no centro Harpers Ferry durante meu terceiro ano. 1 INTRODUCTIONIt is now widely accepted in the conservation world that cellulose nitrate is an inherently unstable material (Horie 1987; Selwitz 1988). Cellulose nitrate–based adhesives are clear and strong when first applied, but they severely discolor and embrittle in time with loss of plasticizers (Koob 1982). Light and heat accelerate the deterioration of the adhesive. Even the slight vibration of a wheeling cart may cause the aged adhesive to fail. Cellulose nitrate has weak adhesive force between the adhesive film and the ceramic but strong cohesive forces that enable the adhesive film to hold together. When the adhesive force between the cellulose nitrate and the ceramic fails, the break edge is often damaged. If the adhesive fails and the vessel is in pieces, it is difficult and timeconsuming to reassemble. For this reason, the archaeological ceramic likely will not be reassembled and therefore will become less valuable to a study collection, since its form cannot be appreciated. This problem of vessels previously reconstructed with cellulose nitrate deteriorating in storage has been experienced in many collections around the country. The idea behind the method of adhesive replacement described here is to stabilize these vessels while they are still whole with a more reliable adhesive. In 2000, approximately 30 million of the artifacts in the National Park Service's collections were classified as archaeological, several million of them ceramics (National Park Service 2000). From the 1930s to the 1950s, many major excavations took place around the world. Thousands of vessels were reconstructed with cellulose nitrate and were placed in museum storage facilities, where they remain. In many cases these vessels were reconstructed quickly, due to the great quantities of objects. Cellulose nitrate was often applied thickly and excessively, possibly in an effort to make reconstruction faster. The adhesive replacement treatment arose from the evidence of the failure, over time, of cellulose nitrate as an adhesive. This effect was seen during an examination of the storage drawers at Colonial National Historical Park that previously contained whole vessels and now contain shards (fig. 1). 2 TREATMENTThe customary method for treating ceramics bonded with an unstable adhesive involves preparation of a map of the reconstruction, complete disassembly of the vessel, painstaking removal of adhesive from each shard, and reconstruction of the vessel with a more stable adhesive. Depending on the size of the object and the complexity of the breaks, this process can take from 8 to 40 hours or more. When considering the magnitude of archaeological ceramics in many museums, this task seems unduly burdensome and expensive. The strategy behind the adhesive replacement method is for the object to remain whole during treatment. Using this method, the majority of the cellulose nitrate is systematically wicked out of the join interfaces with acetone and replaced with Paraloid B-72 (formerly Acryloid—ethylmethacrylate copolymer). Only one section of the vessel is worked on at a time, allowing the whole object to remain intact throughout the treatment. Cellulose nitrate is readily soluble in acetone. This solvent can be obtained easily and inexpensively and is among the least toxic of commonly used solvents. Paraloid B-72 was chosen as the adhesive for this technique due to its reputation as a good adhesive for ceramics, its ease of use, and its long-term stability. Paraloid B-72 is not as hard or as brittle as cellulose nitrate and therefore is better able to tolerate the stresses on the ceramic joins (Koob 1986). Greg Byrne, objects conservator at the National Park Service laboratory in Harpers Ferry, West Virginia, first performed the adhesive replacement treatment in 1986. The object treated was an eastern Native American porous, coarse, unglazed earthenware pot from Colonial National Historical Park. The method used in 1986 was fairly similar to the method illustrated in this article, although polyvinyl acetate (PVAC) AYAF (a high-viscosity, 80 centipoise, thermoplastic resin with a Tg of 24�C) was the adhesive chosen for replacement. Twelve years later, in 1998, the vessel was sent to a private conservation laboratory for examination to determine its stability with regard to potential travel. The object was examined visually through a stereo microscope and physically
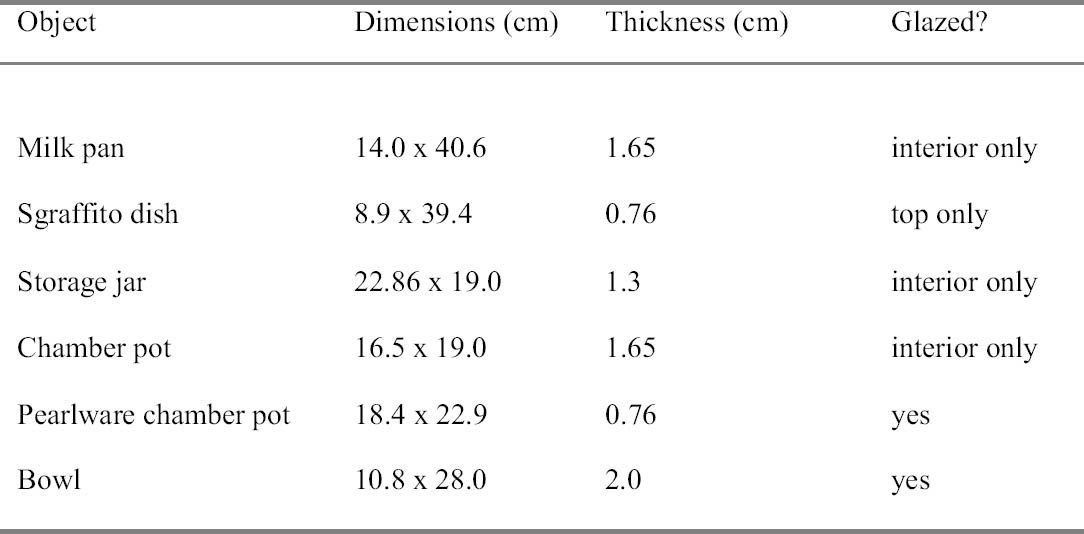
Six objects from Colonial National Historical Park, Jamestown, Virginia, were used to test this method (table 1). The stability of the ceramics before treatment was measured—visually by noting gaps, fills, losses, and flaking surfaces, and audibly by lightly tapping a vessel in several areas and noting the tonal clarity or lack thereof. The reconstructed objects consisted of approximately 15–30 shards with some voids and plaster fills present. With the exception of a single pearlware object, all were earthenware vessels of medium porosity with a light brown/orange clay body. The European pearlware vessel was thinner with a less porous, white body. Overall, the old adhesive had been applied very carelessly, with wide bands extending over the break edges and drips scattered throughout (fig. 2). The vessels were examined and photographed before and after treatment under ultraviolet illumination. Because cellulose nitrate fluoresces bright yellow under ultraviolet illumination, it was easy to see the adhesive within and surrounding all the joins. 2.1 PREPARATIONIn preparation for treatment, the practitioner should have a table set up with fume extraction, a method for supporting the object, two bottles of acetone labeled “clean” and “dirty,” several brushes, cotton, paper towels, a flashlight, a bottle of distilled water, and 30% Paraloid B-72 in ethanol: acetone (3:1). Acetone alone is used to dissolve the CN; it works fast, evaporates quickly, and is less likely to drive the CN into the clay body. Ethanol: acetone (3:1) is the solvent system for Paraloid B-72. The acetone solubilizes the Paraloid B-72 and the ethanol
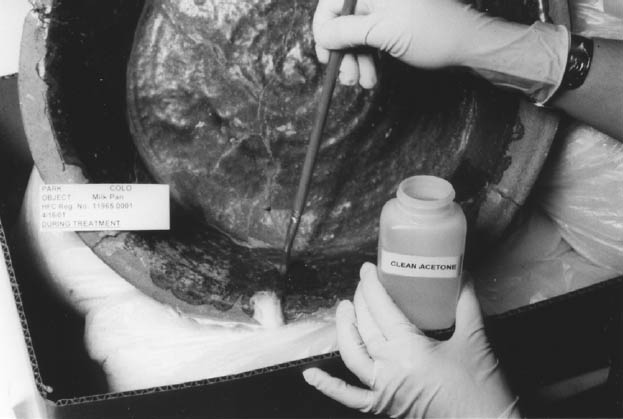
Distilled water was liberally applied by brush to the ceramic on each side of the join to be treated. If salts are present in the ceramic, an alternative nonaqueous solvent would be more appropriate than water for this purpose. Pretreating the site with water filled the porous ceramic and thereby limited the intrusion of the solubilized adhesive solution during solvent treatment (Buys and Oakley 1993). This water barrier method proved effective in preventing tidelines around the breaks on the objects in this study. Having glaze present on one or both sides of the ceramic naturally reduces the possibility of tidelines. Next, a wad of long-fiber cotton was stretched into a strip approximately 1.0 cm wide and placed on the exterior of the vessel along the length of the first join to be treated. The purpose of the cotton was to absorb flushed-out solvent and old, yellow adhesive. The cotton wads were systematically replaced when they became saturated, until the join was free of adhesive. 2.2 REMOVAL OF CELLULOSE NITRATEIt is best to begin with the tightest joins first. If significant gaps exist between the shards, they can be minimized toward the end of the process, while the adhesive is slightly soft. Each object is a puzzle, and with practice, one sees which pieces are more or less crucial in holding the vessel together. Not concentrating in one area proved most effective in moving quickly around the object while keeping it in one piece. With a soft, medium-sized brush, acetone is liberally applied along the inside of the selected break edge (pretreated with water from the previous step), flushing the join with solvent. Old cellulose nitrate is easily soluble in acetone, and the adhesive is quickly wicked into the cotton wadding in place on the other side of the join and is also lifted away with the brush. The brush is wiped clean on a paper towel and rinsed in a separate bottle of acetone labeled “dirty.” With the acetone from the “clean” bottle, the brush is used again to apply acetone to the cellulose nitrate–filled join and to lift out excess adhesive. A brush was found to be more useful than a pipette or a syringe, as it not only introduced the solvent but also lifted out the old adhesive. A significant amount of adhesive can be removed by the brush, therefore limiting the amount of solubilized adhesive that passes into the ceramic. Continually flushing the join with acetone quickly saturated the cotton wadding. The cotton released easily from the vessel in a tidy, soaked strip and was replaced until the acetone flushed clear and no trace of yellow adhesive was wicked into the cotton. Cotton linters stuck to the ceramic were not a problem due to the substantial amount of acetone present. When very tight joins needing treatment were encountered, the same method was employed. However, it was more difficult to determine at what point the old adhesive has been flushed from the join. Both a flashlight and a fiber optics light source were useful tools in determining when the join was free of adhesive (fig. 3). At this stage there is the opportunity to clean up any excess cellulose nitrate that may be found on the surface of the vessel. The adhesive replacement treatment is not meant for cosmetic purposes, but, when appropriate, this can be an important step and does not take much extra time. A skin of brittle adhesive on a glazed surface is likely to flake, often taking the glaze with it, resulting in significant damage and loss of information. Several of the objects in this study had sloppily applied adhesive on their glazed surfaces that was brittle and was causing the glaze to flake. Using the brush and acetone, the adhesive can be removed or reduced, and the glaze flake can be saved from detachment. The cellulose nitrate can be reduced and manipulated to tack down the glaze flakes until Paraloid B-72 can be wicked in during the following treatment stage.
One of the objects treated contained an old plaster fill. This fill was stable and firmly attached to the shards adjoining it, so it was not removed. Again, it must be stated that this treatment is purely for stabilization purposes. Gaps or voids may be present between shards after treatment, but as long as there is good contact between most of the surfaces, the shards will remain in place. 2.3 ADDITION OF B-72When the acetone and the brush remain clean and there is no visual trace of old adhesive, a generous amount of Paraloid B-72 (30% by weight) in ethanol 3 RESULTS AND DISCUSSIONThe customary disassembly-reassembly method and the adhesive replacement method can both be employed to stabilize the structure of an old ceramic restoration. Aesthetically, the argument can be made that the traditional method produces a more appealing object than the adhesive replacement method. However, because the traditional method is so laborintensive and time-consuming, many objects, especially the multitudes in study collections, often go untreated. If the goal of retreatment is to stabilize a large collection of stored or study collection objects, then the adhesive replacement method seems to provide a cost-effective alternative in the reconstruction of an unstable vessel by avoiding the time-consuming disassembly process. Also, removing the excess embrittled cellulose nitrate flakes from the surfaces around the failures can both help preserve information by preventing the brittle flakes from breaking off fragile surfaces and markedly improve the object's appearance. Other than the pearlware chamber pot, the objects treated in this survey were fairly porous. Based on the effectiveness of this treatment on the pearlware vessel and on entirely glazed vessels, it is the author's belief that it would be effective on more impermeable ceramics and possibly even glass. With less porous materials, the solvent evaporation of the Paraloid B-72 would be much slower due to its inability to escape into the clay body. More support for the vessel would likely be necessary during treatment. The less porous the material, the lower the possibility of tidelines and the greater the chance of removing more of the original adhesive. Following this train of thought, less porous glazed vessels can be seen as particularly good candidates for this treatment. Glaze can act as a barrier, forcing the acetone through the break and not as readily through the clay body. Glaze on both the interior and exterior of porous ceramics may inhibit full water soaking of the body before treatment. On porous glazed ceramics, the acetone may still be able to enter the clay body, other than solely at the join, through pits, discontinuities, or crackle patterns. In these cases, the acetone may dissolve the cellulose nitrate and move it into the clay body and under the glaze. Since glaze impedes materials from escaping porous bodies, caution and testing should be practiced before treating these objects. On the unglazed surface of one of the objects treated, a darkened tideline was noticeable after the treatment was complete. However, this was the first object treated, and water had not been used to pretreat the joins. The author acknowledges that the creation of tidelines is a possibility with this treatment. When considering the options, though, a tideline is less potentially damaging to an object than the collapse of the form or the loss of glaze due to brittle adhesive. In the example treated, the resultant tidelines were far less unsightly than the original excess adhesive. 3.1 TIME SAVINGSBecause no two archaeological ceramic objects fail in exactly the same manner, it is not possible to directly compare the time required to stabilize objects by the traditional disassembly-reassembly method and the adhesive replacement method. In an effort to gain some relative sense of how the two compared, a colleague unfamiliar with adhesive replacement 3.2 STABILITYSeventy-two hours after treatment, the six Jamestown vessels were reexamined by sight and sound for stability. Visually, the gaps, fills, losses, and flaking surfaces appeared to be stable. A more reliable auditory indicator of increased stability was the clear tonal ring produced by gentle tapping. It has now been three years since the six vessels were treated. All the objects remain stable by these same indications. Sixteen years have passed since the first vessel was treated with AYAF. As mentioned in section 2, this vessel was recently examined and determined stable enough for travel. Considering that the adhesive in the joins of the re-treated vessels are likely a mixture containing some cellulose nitrate, one could draw the following conclusion: Since the vessel re-treated with AYAF remained strong after 16 years—even though it may contain some residual cellulose nitrate—we can assume that the vessels treated with Paraloid B-72 will remain strong, owing to B-72's proven long-term stability and good aging characteristics. 3.3 FURTHER ANALYSISThere are many scientific and material property issues that could not be addressed in the scope of this work. The occurrence of adhesive mixtures is an issue in all branches of conservation, yet it is rarely studied or discussed. Research on adhesive mixtures in a systematic manner is ongoing at the National Park Service. At Harpers Ferry Center, Fourier transform infrared spectroscopy (FTIR) was performed on adhesive samples taken from three of the joins of the original treated vessel. Two samples contained only AYAF and one contained some cellulose nitrate and some AYAF (Bischoff 2002). This study indicates that although most of the cellulose nitrate is likely removed, some may remain. The stability of a mixture of Paraloid B-72 and aged cellulose nitrate—that is, the effect of residual plasticizers in the deteriorated cellulose nitrate on Paraloid B-72—is a topic for consideration. Other potential topics for further study are determining adhesive concentrations in mixtures and how they relate to mechanical strength, and what types of ceramics or glass are best suited to this treatment and which are not. What is the result of solubilized adhesive in the clay body? Could this actually help to consolidate the edge? The author invites colleagues in the conservation community to try out this treatment and report back with their results. 4 CONCLUSIONThe purpose of this article was to present a new treatment strategy for archaeological ceramics. Considering the examples chosen for this study, the treatment was successful in achieving the goal of replacing an unstable adhesive with one that appears to be much more stable and doing this in a fraction of the time required by more traditional treatment methods. In addition, the object's appearance was improved, and valuable information was maintained. Precautions should be taken when embarking on this treatment. Some objects might not be appropriate for this treatment, including those with solventsensitive surface treatments, extremely porous vessels, or those that contain soluble salts. Although darkening or white tidelines are a possibility, they were not witnessed in this treatment except when water was not used as a pretreatment barrier. Visual inspection under normal and ultraviolet illumination demonstrated that the vast majority of the cellulose nitrate adhesive was removed from the joins, as well as from the interior and exterior of the vessels. While performing this treatment, it is difficult to know exactly how much cellulose nitrate remains, but it is clear that enough can be removed to allow for the addition of Paraloid B-72 to achieve a strong join. Paraloid B-72 is regarded as a highly stable adhesive and is the suggested adhesive for archaeological ceramics (Koob 1986). The solubility of both Paraloid ACKNOWLEDGEMENTSThe author would like to thank Greg Byrne for encouraging me to pursue and refine this technique. Overall thanks go out to all at the National Park Service laboratory in Harpers Ferry, and to all my friends and colleagues who supported and backed me in this effort. REFERENCESBischoff, J.2002. Personal communication. Conservation Science Laboratory, National Park Service, Harpers Ferry, W. Va. Buys, S., and V.Oakley. 1993. Conservation and restoration of ceramics. Oxford: Butterworth-Heinemann. Down, J., M. A.MacDonald, J. T�treault, and R. S.Williams. 1996. Adhesive testing at the Canadian Conservation Institute: An evaluation of selected poly(vinyl acetate) and acrylic adhesives. Studies in Conservation41(1):19–44. Horie, C. V.1987. Materials for conservation. London: Butterworths. Koob, S. P.1982. The instability of cellulose nitrate adhesives. Conservator6:31–34. Koob, S. P.1986. The use of Paraloid B-72 as an adhesive: Its application for archaeological ceramics and other materials. Studies in Conservation31:7–14. National Park Service. 2000. FY2000 Collection Management Reports, Museum Management Program, Washington, D.C. Harper's Ferry, W. Va.: National Park Service. Selwitz, C.1988. Cellulose nitrate in conservation. Los Angeles: Getty Conservation Institute. FURTHER READINGShashoua, Y., S. M.Bradley, and V. D.Daniels. 1992. Degradation of cellulose nitrate adhesive. Studies in Conservation37(2):113–19. SOURCES OF MATERIALSParaloid B-72Talas 568 Broadway New York, N.Y. 10012 (212) 219-0770 www.talasonline.com Chemicals (acetone and ethanol)Sigma-Aldrich, Inc. 1001 W. St. Paul Ave. Milwaukee, Wis. 53233 (800) 325-3010 www.sigma-aldrich.com Absorbant Sanitary Bulk CottonFisher Scientific 4500 Turnberry Dr. Hanover Park, Il. 60103 (630) 259-1200 (800) 772-6733 www.fishersci.com AUTHOR INFORMATIONMICHAELA NEIRO is assistant conservator at the Society for the Preservation of New England Antiquities. She received her master's degree in objects conservation from Buffalo State College in 2000. She continues to pursue treatments and research on both organic and inorganic objects. Address: SPNEA, 151 Essex St., Haverhill, Mass. 01832
 Section Index Section Index |