DOES LOW-TEMPERATURE PEST MANAGEMENT CAUSE DAMAGE? LITERATURE REVIEW AND OBSERVATIONAL STUDY OF ETHNOGRAPHIC ARTIFACTSELLEN CARRLEE
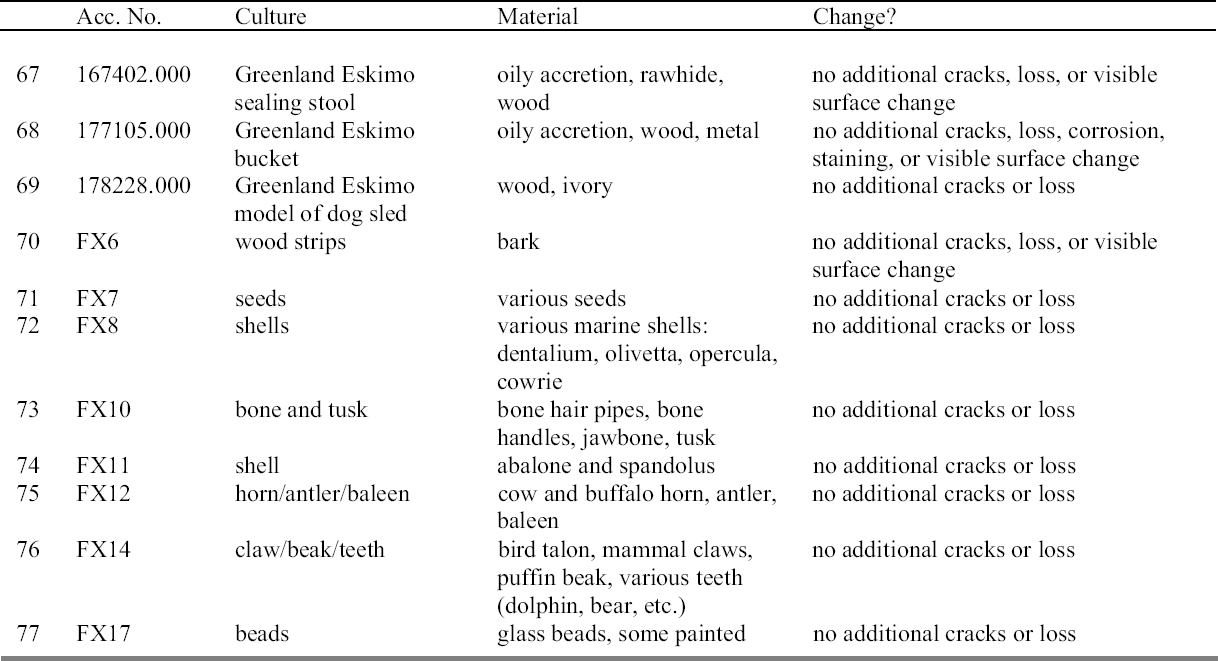
6 CONCLUSIONSThe observational study of vulnerable ethnographic material frozen at NMAI indicated no structural failure or visible surface changes to the objects. Low-temperature treatment conditions for this study were more extreme than recommended, in terms of both temperature and duration. Further evidence that encourages a cautiously optimistic attitude toward freezing can be found in several articles addressing museum storage in cold climates (Gates and Thorp 1982; Lafontaine 1982). So-called “humidistatically controlled heating” aims to control the RH of museum storage buildings in northern climates during the winter months, based on the principle that the RH of a constant volume of air with a given moisture content can be controlled by adjusting the temperature. No damage to objects has been reported, although vulnerable materials do not tend to be stored in these buildings. It is important to make the distinction between open systems such as these and the more controlled closed systems achieved with a sealed bag in low-temperature pest control. Exploration of the literature and consideration of materials science issues raise two areas of concern. One involves the likelihood of repeated freezing cycles for some objects, particularly those actively loaned or exhibited and therefore subjected to low-temperature treatments with each re-entry into the museum collection. Experiments involving wood (Starecka and Mieczyslaw 1986; Erhardt et al. 1996), textiles (Dawley 1993; Holt et al. 1995; Jansson and Shishoo 1998; Peacock 1999), paper (Bj�rdal 1998), synthetic fishing gear (Toivonen 1992), and insect collections (Rawlins 2001) suggest no significant structural damage occurs with repeated freezing cycles for pest control. It has been asserted that repeated cycling within the range of plastic deformation has no structural effects on most museum objects (Tumosa et al. 1996). Herbaria and natural history museums seem to have more of a tradition of successfully freezing collections on a regular basis than fine art museums do (Florian 1990b;Tanimura and Yamaguchi 1995; Shchepanek 1996;Ackery et al. 2000). One of the most encouraging examples comes from entomologist John Rawlins of the Carnegie Museum of Natural History, where 22,000 drawers of insect specimens are preventively exposed to –28�C for two cycles every 18 months with no observed damage to pigments or structure and no
The second area of concern involves the permanent physical changes that theoretically could occur (and perhaps accumulate) on a molecular level but remain invisible to the naked eye, such as loss of strength, loss of elasticity, distortion, crystallization, molecular alteration, and denaturation. In some cases there may be synergistic effects in which interrelated damage mechanisms combine to cause further problems. While low-temperature treatment remains the best solution when ethnographic artifacts are actively infested, the need for preventive low-temperature exposure for objects entering and re-entering the museum environment is less obvious. Museum staff must weigh the potential risk for devastating loss through insect damage against the possible cumulative damage posed by repeated low-temperature exposure. Responsible prevention must include consideration of factors such as the quality of integrated pest management in the exhibit space or requesting institution, packaging and shipping conditions, duration of possible exposure to infested environments, ability of staff to perform adequate visual inspection, and the susceptibility of artifact material to insect pests. ACKNOWLEDGEMENTSMany thanks to Dr. Dana Elzey of the Department of Materials Science and Engineering at the University of Virginia, and the staff of NMAI, including Marian Kaminitz, Emily Kaplan, Jessie Johnson, and Leslie Williamson, for their support and feedback. I would particularly like to acknowledge Mary Lou Florian for her excellent work on this topic. Thank you to the Andrew W. Mellon Foundation for making this research possible. |