DOES LOW-TEMPERATURE PEST MANAGEMENT CAUSE DAMAGE? LITERATURE REVIEW AND OBSERVATIONAL STUDY OF ETHNOGRAPHIC ARTIFACTSELLEN CARRLEE
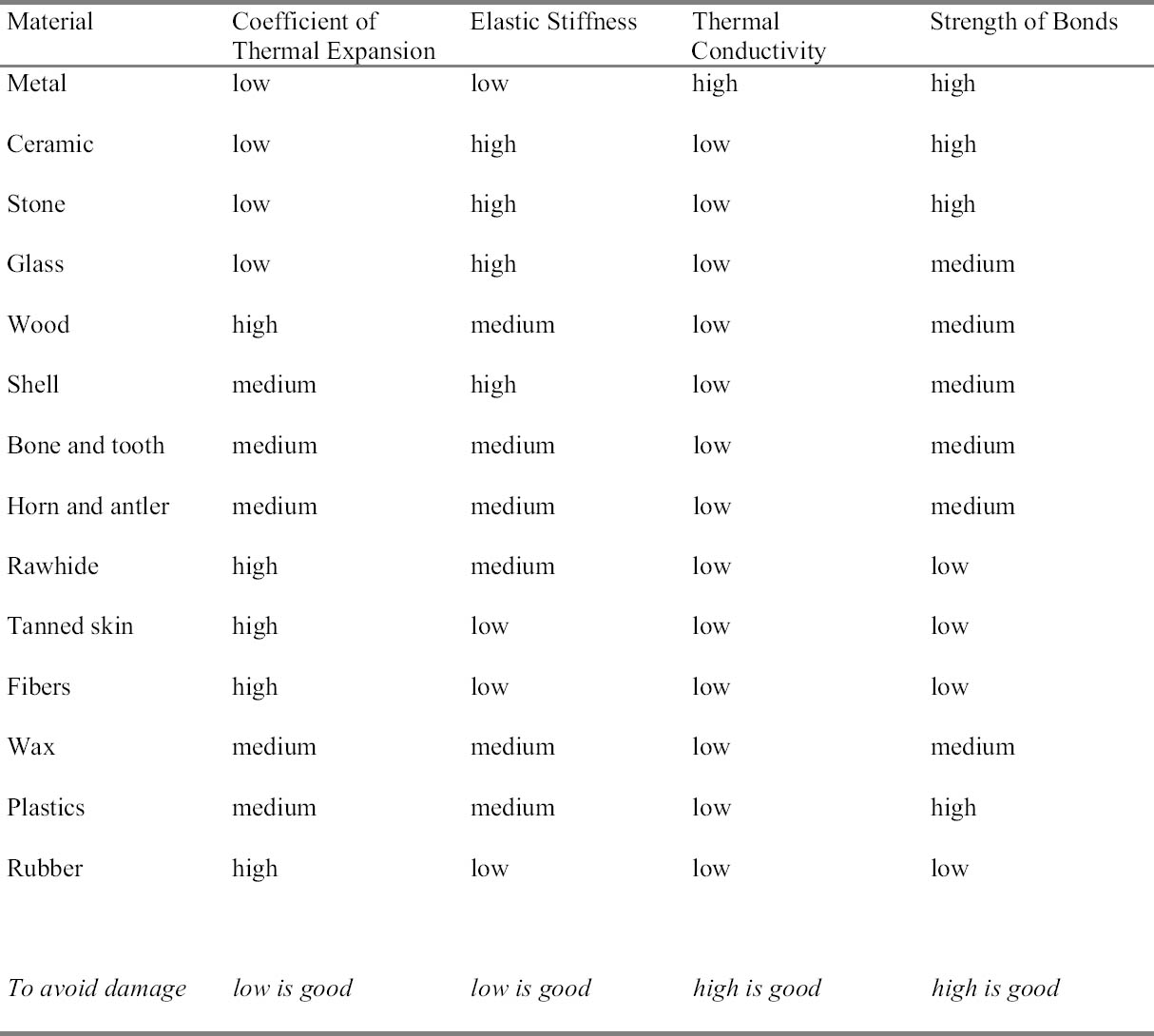
2 CONCERNS FOR MATERIAL CHANGES FROM FREEZINGSeveral categories of physical change were considered during this study to determine what mechanisms might be responsible for potential damage to artifacts during low-temperature treatment. References for low-temperature damage found in several nonconservation fields proved tantalizing but difficult to apply to the museum situation. The fields of cryogenics (the study of living systems at low temperatures) and low-temperature physics utilize a temperature range much colder than proposed for museum pest control. In general, Arctic studies, refrigeration engineering, and the food preservation industry deal with considerably more free water than pertains to the museum treatment situation. Freeze-thaw, dehydration, condensation, swelling, embrittlement, shrinkage, thermal shock, polymorphic phase change, and molecular alteration have all been mentioned by conservators as possible areas of concern. Some of these are relevant to museum pest control, and others are not. The field of materials science suggests factors of greater relevance. 2.1 MOISTURE AND HUMIDITY ISSUESLittle information is available to conservators about potential damage to museum objects from low temperatures. Most concerns about the treatment are theoretical, extrapolated from the extensive information available about the reaction of museum objects to changes in relative humidity and from empirical evidence from daily life (such as one's home freezer). Some of these assumptions are incorrect. In a museum treatment situation using proper packaging, most objects lack sufficient moisture for freeze-thaw or dehydration mechanisms to occur. Inclusion of adsorbent buffering materials and the counteraction of shrinkage mitigates swelling as a concern, while proper bagging eliminates the danger of condensation on the object and creates a closed system. Conservation scientist Mary Lou Florian of the Royal British Columbia Museum has written extensively about moisture relationships and proper low-temperature protocol and included lengthy bibliographic references outside the field of conservation in her articles (Florian 1986a, 1986b, 1987, 1990a, 1990b). 2.1.1 Freeze-ThawUse of the term “freezing and thawing” to describe the museum pest control process should perhaps be replaced with the more accurate “warming and cooling.” The term “freezing” is loosely used in the conservation literature to imply temperatures below 0�C, and in this context does not necessarily imply a phase change from liquid (water) to solid (ice). Review of the scientific literature indicates ice does not tend to form in museum objects. A brief review of water dynamics is helpful in understanding several issues surrounding potential damage to artifacts. Moisture content (MC) is the percent weight of water in relation to the dry weight of the material. Water activity (Aw) reflects the portion of water within the moisture content that can be used for “activity” such as chemical reactions, availability to microorganisms, or exchange of humidity between the material and its environment. Water activity is given as the ratio of vapor pressure of water in a material compared to pure water under identical conditions. Sometimes this number is multiplied by 100 and called the equilibrium relative humidity (%ERH). Equilibrium moisture content (EMC) is a measure of the amount of water in an object after it has reached equilibrium with its surroundings over time. Water can exist in many physical states on a continuum reflecting how strongly the water is bound to another material. On one extreme, it can be tightly bound in a single layer to the polar sites of molecules. This water does not freeze (Nomura et al. When it does occur, ice formation is commonly known to cause freeze-thaw damage from the 9% expansion in volume that takes place as water changes from the liquid to the solid phase (Franks 1985). Other phenomena, however, are also related to the formation of ice. Living plants hold their shape in part due to “turgor pressure” or the pressure of water inside the cells of the plant tissues. At low temperatures, an increase in the permeability of the cellular plasma membrane (due to pressure from the 9% increase in volume as ice crystals grow) causes a loss of cellular water, thus turgor pressure and the subsequent “wilted” appearance of some frozen plant materials (Reid 1987). However, the most damaging problems associated with freezing are the “concentration effects.” As an aqueous solution freezes, water separates out of the mixture as ice, and the concentration of the remaining solutes increases (Taylor 1987). This result affects a variety of factors within the cell, including ionic strength, viscosity, oxidation-reduction potential, pH, salt concentration, and enzyme reactions. The majority of the literature associated with freezing damage focuses on these effects (Hawthorn 1968; Poulsen and Lindelov 1981; Kobs 1997). At low-moisture-content levels such as those present in most museum objects, the possibility of ice formation, loss of turgor pressure, and damage related to concentration effects is eliminated. 2.1.2 DehydrationDehydration does not occur in objects inside the freezer if there is insufficient free water to be lost (Florian 1986b; Strang 1997). The ability of air to hold moisture is temperature-dependent. The word “relative” in “relative humidity” refers to the relationship of moisture present to the maximum of it that air can hold at a given temperature. Wintertime air, for example, is drier than summertime air because cool air cannot hold as much humidity as warm air. When dry winter air is warmed inside a building, it is able to hold more humidity, and inhabitants of that building (including food, plants, and people) help provide the moisture to bring the air into equilibrium at a higher RH. Complaints of dry, itchy skin in the winter are often related to the ability of human skin to provide water for warmed, dry air “hungry” for moisture. The cold air inside the freezer is not “hungry” for moisture. The equilibrium moisture content in an adsorptive object actually tends to increase due to the decreased ability of the air to hold moisture. The relative humidity measured in freezer-bagged enclosures does not correlate to the experience of the object at the same RH in a standard room temperature situation. A reading of the RH measured in the enclosure must take into account the moisture the air has already given up to the object and buffering materials. In this case, the lower RH does not signify a situation where the object is being desiccated by a dry environment. On the contrary, reducing the temperature allows the object to slowly A review of the process called “freeze-drying” is helpful in understanding why museum objects do not dry out during low-temperature pest control. Freeze-drying is a process involving the removal of frozen water from an object by sublimation. In contrast to evaporation, where liquid water is turned to water vapor and carried off, sublimation is the removal of water vapor directly from ice without its becoming liquid. “Freezer burn” is the surface dehydration of poorly packaged foods caused by sublimation in the freezer. Freeze-drying is a more sophisticated process involving a vacuum in order to take advantage of the properties of water under low pressure. Under low pressure, water will vaporize at a lower temperature. That is, water does not need to be as hot to become water vapor. (This circumstance is related to the phenomenon of water boiling at a lower temperature at a high elevation due to the lower atmospheric pressure of thin mountain air.) With the use of a cold condenser and a gentle heating element, the air in the freeze-drying chamber is kept at a temperature slightly above the temperature of the frozen object, allowing water molecules on the object's surface to break free and gather on the condenser as frost (Schmidt 1985). This treatment is used only when museum objects are wet and have sufficient free water to form ice and allow sublimation to occur. 2.1.3 CondensationCondensation is a result of the reduced capacity of air to hold moisture as its temperature is lowered. It is this phenomenon that is observed on a car's windshield in the winter. When the interior of a car is warmed, the warm air encounters the cold wind-shield, and a microclimate of cold air is created near the surface. This small cushion of cold air cannot hold the same level of humidity held by the warm air, and moisture condenses on the interior of the windshield. Turning on the air vent next to the windshield will alleviate the problem, moving the warm air away from the surface before it has the opportunity to cool. Some artifact materials, such as wood and hide, are able to adsorb the humidity released by the air at low temperature and release it again when brought slowly to room temperature. Nonadsorbent materials, such as metals and stone, do not have that capacity and are vulnerable to condensation on their surfaces when cooled.(Frost is simply frozen condensation.) Condensation could potentially cause staining, migration of colorants, corrosion, or fungal growth. Addition of adsorbent packing materials such as crumpled tissue reduces the likelihood of condensation on nonporous objects, as adsorbent materials act as a buffer and adsorb available water vapor. Placing the objects in sealed plastic bags with most of the air removed reduces the amount of moisture available in the air for possible condensation. The sealed plastic bag also serves to prevent condensation on the cooled object as it returns to room temperature. Any condensation during warming would form on the outside of the plastic bag, following the model that condensation forms on the warm side of the warm/cool interface. Although polymer films, such as polyethylene bags, are slowly permeable to moisture, it occurs over a longer period of time (several weeks) than the bags would be in use for cooling (several days) (Strang 1997; Florian 1992). 2.1.4 SwellingSwelling of materials is another consequence of the reduced ability of cold air to hold moisture. The excess moisture can be adsorbed by porous materials, and a small amount of swelling may take place. Again, this result can be mitigated by the inclusion of buffering materials to sacrificially adsorb and release the excess moisture. Wood, for example, swells at low temperature if it has exposure to open air. However, in the bagged situation, the equilibrium moisture content (EMC) change is not significant because the amount of water available for adsorption in the bag is small in relation to the amount that can be adsorbed (Florian 1990a, 1992). Furthermore, materials at low temperatures take longer to reach moisture equilibrium with the environment than the same materials at room temperature. Photographic films have been reported to take between 10 and 30 times longer to reach equilibrium at low temperature (Adelstein et al. 2.2 PROPERTIES OF MATERIALSThe biggest potential risks faced by museum objects during freezing stem not from moisture-related issues but from the properties of materials at low temperatures and the mechanisms of cold-induced damage. Important factors include the coefficient of thermal expansion, stiffness, thermal conductivity, and strength of the material. Thermal cycling and the magnitude of temperature change can pose potential dangers. Geometry, aging, residual stresses resulting from manufacture, and the history of each unique object can also play significant roles. An understanding of the damage mechanisms will contribute to a more informed consideration of the risks and a logical approach to decision making. 2.2.1 EmbrittlementEmbrittlement occurs at temperatures used for pest control because the molecules are resistant to motion. Increased tendency to fracture is related to this reduced ability to deform. So-called “glassy” behavior occurs when the molecules do not vibrate enough to bump past each other during the application of stress. The “glass transition temperature” (Tg) is the range of temperatures at which molecular motions become slower than the rate at which temperature is changed and the material no longer has sufficient time during cooling to remain in equilibrium. The material then changes from soft and rubbery to solid and glassy with a decrease in specific volume (shrinkage). The Tg is sometimes given as a range because it can be affected by factors such as the age of the material (aging increases the Tg) or rate of cooling (slower cooling gives a lower Tg). Below Tg, brittle fracture can occur by crack propagation. Elastic deformation tends to occur above Tg. Conservators are perhaps most familiar with the concept of Tg in relation to adhesives. AYAA Poly (vinyl acetate) (PVA) adhesive, for example, has a lower Tg than Paraloid B-72, and is therefore more flexible at room temperature and preferred for use in situations where more flexibility in the join is desired, such as repairing feather quills. However, in an archaeological field setting, the low Tg of PVA may cause slumping of reconstructed ceramics. Thus the higher Tg of Paraloid B-72 is preferred. Stiffness describes the amount of elastic deformation resulting from a given applied stress. “Elastic” behavior describes the ability of a material to deform under stress and still return to its original conformation. Objects with a high elastic stiffness tend to be brittle. Stiffening of most elastomers occurs below –20�C, while the brittle point does not begin to occur until –50�C (Sehgal and Lindberg 1973). Examples of materials that become brittle in this temperature range include rubber, resin varnishes, linseed oil films (oil paint), synthetic polymers, acrylic paint, and soft vinyl (Mecklenburg and Tumosa 1991; Michalski 1991). Linseed oil, for example, becomes fully glassy at –30�C (Michalski 1991). Although elastic stiffness induced by cooling is usually reversible with warming, materials are potentially vulnerable to structural damage until sufficiently warmed. Dangers could include vibrational stresses from motors inside faulty freezers, rough handling when moved while cold, and even the weight of the object itself (as in the case of a load-bearing adhesive). Glass is an example of a material with a high elastic stiffness. In addition, glass is a poor conductor of heat and has a low resistance to crack growth. Generation of small surface cracks is likely to occur from cooling during manufacture as the surface goes into tension. Repeated exposure to low temperatures could result in one of two outcomes for these cracks: “ratcheting” or “shakedown.” Ratcheting describes the accumulation of plastic strain. Each time the crack is opened and closed, the crack grows. Damage evolution due to thermal cycling is known as “thermal fatigue” and will eventually lead to macroscopic failure. The other option, shakedown, involves a reduction of the incremental strain per cycle. Most of the damage in this process happens the first time the object is exposed to low temperature, and each subsequent 2.2.2 ShrinkagePractically all materials shrink as temperature is lowered because of the reduced vibration on the atomic or molecular scale. (Think of how gas expands during heating due to the more active motion of its molecules.) The decrease in vibration causes the molecules to have a smaller range of motion and thus to take up less space. The “coefficient of thermal expansion” (CTE) is a measure of this change, expressed as a ratio of change in length per degree Celsius compared with the base length at some reference temperature (cm/cm/�C). How much a material might shrink is dependent on the strength of the interatomic bonding. Objects with strong chemical bonds, such as metals and ceramics, expand and contract less with changes in temperature than objects with weaker bonds. Rabbit skin glue, for example, with a CTE of .000025 cm per degree Celsius, will shrink only 0.1% when cooled from 20�C to –20�C (Mecklenburg and Tumosa 1991). In his discussion of paintings, Michalski cites 3% elongation as the elastic limit beyond which a polymer cracks (Michalski 1991). Low-temperature treatment of composite objects gives rise to a risk of damage due to CTE mismatch if the two materials have different coefficients of thermal expansion. Internal stress, deformation, and damage could occur as the composite object is heated or cooled. There are published tabulations for expansion coefficients of some common materials, but there may be no data for many materials in aged or altered condition, or no data in the appropriate temperature range, or simply no data at all. Often materials are simply categorized as high or low relative to each other. During cooling, the low CTE material goes into tension while the high CTE material is in compression and in danger of cracking or delaminating. CTE differences or mismatch can also be seen within a single material, particularly those that demonstrate anisotropy. The bonds in anisotropic materials are direction-dependent and expand to different degrees in different directions. Examples include materials with a complex structure that tend to crack in a preferential direction, such as wood, bone, tooth, and lamellar structures. Since the rate of freezing does not affect the CTE mismatch, it is not possible to mitigate damage by controlling the rate of freezing. Cracking is not the only manifestation of CTE mismatch. If the high CTE material is more vulnerable, deformation or crushing may occur as the material goes into compression. A high CTE material sandwiched between two layers of low CTE material may be extruded by pressure from the surrounding material. At sufficiently high stress, materials lose their ability to deform elastically, resulting in either failure or plastic deformation. Unlike elastic deformation, which is fully recovered when the applied stress is removed, plastic deformation is permanent. Plastic deformation of capillary structure and loss of water bonding sites is thought to contribute to the loss of moisture-regain ability in skins and furs exposed to cold storage (Pool 1997). Plastic deformation of cell structure and subsequent depletion of gas is thought to contribute to observed Ethafoam shrinkage in the freezer. The inability of air to replace the lower molecular weight gas that may have been squeezed out of the individual Ethafoam cells may also be a factor (Elzey 2001). Low RH is a far more common cause of shrinkage in artifacts than the effects of low temperature alone (Michalski 1991). 2.2.3 Thermal ShockThermal shock is the condition resulting when rapid temperature change leads to excessive internal stress resulting in damage or failure. It is the phenomenon that occurs when cold water is poured over a hot ceramic plate, causing it to shatter. The magnitude of the stress is determined by the overall change in temperature, the rate of cooling, the size of the object, and the material's CTE, elastic stiffness, conductivity, and strength. Materials with high CTE, high elastic stiffness, low thermal conductivity, and
2.2.4 Polymorphic Phase ChangePhase change refers to a change in state, such as from a solid to a liquid, or liquid to gas. “Polymorphic” phase change implies a change from one solid state to another, often seen in metals at elevated
Observation of fatty bloom on a dressed leather saddle treated for pest control suggests that polymorphic phase changes might occur in museum objects at low temperature (Baughman 1999). Leather dressing often includes Neat's foot oil from which the solid triglyceride portion has been removed through chilling, causing the solids to rise to the surface of the oil. Solid triglycerides remaining in Neat's foot oil dressing may cause spew at low temperature (Fogle 1985). Rubber is another material reported to undergo changes at low temperature. The rate of crystallization of rubber increases with decreasing temperature, reaching a maximum at approximately –25�C. Rubber that is crystallized is characteristically inelastic and may have hard or “crunchy” cracked surfaces. This alteration is sometimes reversible upon warming (Baker 1995). Allotropes are polymorphs of elements, and some occur at low temperature. Tin disease (tin pest, tin blight, tin plague) is one such example. One pure tin allotrope, beta tin, is the shiny stable white metal seen at room temperature. The alpha tin allotrope (a nonmetallic crumbly gray powder) becomes the more stable form as temperatures decrease, reaching a maximum at –30�C. Upon warming, crystalline faults form, exacerbating the problem (Elzey 2001). Tin disease is inhibited by as little as 0.1% bismuth, antimony, or lead, the typical alloying metals used with tin. Most of our museum materials (such as tin cone tinklers found on Native American artifacts from the Great Plains) are alloys and therefore safe from polymorphic phase change in the freezer. However, the textbook example of tin disease involves Napoleon's attempted 1812 winter invasion of Moscow, which failed in part because of the disintegration of the tin buttons on the soldiers' clothing. 2.2.5 Molecular AlterationThe technology for studying proteins at low temperature in the absence of ice formation has been developed only within the past decade. Previously, scientific knowledge of low-temperature “denaturation” or unfolding of proteins was based on extrapolations from high-temperature experiments. Current research indicates that the denaturation of proteins has different causes at high and low temperatures and results in different disruptions of the molecule (Fahy 1995; Franks 1995). “Low temperature” studies of biological phenomena rarely involve temperatures below –70�C and often involve temperatures just below –0�C (Douzou 1977; Taylor 1987). Conformational stability in proteins is dependent on a complex energy balance involving a variety of intermolecular forces. Cooling weakens some forces, such as hydrophobic interactions, but enhances others, such as hydrogen bonding. These kinds of changes in the molecule may not be completely reversible upon warming and could alter some of the identifying characteristics of the protein (Taylor 1987). Many of these studies, however, involve freezing proteins with significant moisture content and suffer from the associated concentration effects. Simple exposure to low temperature exclusive of moisture-related complications Although the rate of most chemical reactions tends to decrease with decreasing temperatures, according to the Arrhenius equation (Mills and White 1987), oxidation of lipids is an important exception. Autoxidation of unsaturated fatty acids, however, can be accelerated by low temperatures in the range used for pest control (Franks 1985). Autoxidation of lipids in foods is associated almost exclusively with unsaturated fatty acids such as are found in vegetable oils (Karel 1985). Lipids contain a wide variety of fatty acids that differ in chemical and physical properties as well as in their susceptibility to oxidation. Lipids in the NMAI collection include animal fats, plant waxes, beeswax, avian preen oil on feathers, and lanolin in wool. These materials have complex combinations of lipids that usually include a percentage of unsaturated fatty acids. The autoxidation of saturated fatty acids is very slow and slower still at low temperature. Oxidation may also be catalyzed by enzymes, although the definition of enzymes as “proteins produced by living organisms functioning as biological catalysts in living organisms” (Roberts and Caserio 1977) calls into question whether there are any active enzymes remaining in museum objects. Furthermore, some enzymatically catalyzed oxidation in lipids is influenced by solute concentration effects that allow enzymes and substrates to come into contact (Karel 1975; Reid 1987). Museum objects that cannot form ice are unlikely to face these problems. Caution must be exercised in extrapolating data from other fields. Agricultural research, for example, is concerned with the longevity of biological tissues as a nutritional resource and issues such as flavor and texture preservation. The behavior of fresh fish muscle at low temperatures and its purpose upon thawing is very different from the behavior of an aged, dried fish skin artifact. The cryogenics field is concerned with the viability of tissues at low temperatures in the colder range of –80 to –196�C (Reid 1987). Both fields focus closely on the continuation of biological function and address issues of decomposition and cell death from a point of view that considers loss of structural integrity a somewhat secondary concern. For these fields, their objectives have already been lost at the level that museum freezing for pest control is addressing. The loss of moisture-regain ability due to changes on the molecular level is another realm of potential problems for organic materials (Kronkright 1990; Pool 1997). The term “hysteresis” is used to describe nonlinear input-output systems because of material memory. Imagine an experiment measuring water content at different relative humidities. The experiment could be set up in two ways: the material may begin dry and measurements taken as it adsorbs water, or the material may begin wet and measurements taken as it desorbs water. Interestingly, at a given RH, the water content of the material is higher when it is in the process of desorbing than when it is in the process of adsorbing (T�mar-Bal�zsy and Eastop 1998). This finding is thought to be because polymers are at a more stable energy state with higher moisture content and are not as willing to give up moisture as they are to take in moisture. This phenomenon of adsorption and desorption rates relying on moisture history is one example of hysteresis. At low temperatures, molecules with potential water-holding sites may draw closer together and bond, creating a reduced capacity to hold water in the future. Water activity and moisture content are related by a curve known as a “moisture sorption isotherm.” It has been reported that sorption ability decreases with increase of cold storage time (Wolf et al. 1972). Long-term cold storage of furs and skins in an open system (not in a sealed bag) has been reported to cause a loss in moisture-regain ability (Pool 1997). Another study suggests that low-temperature treatment for pest control (a comparatively brief period of time) has a minimal initial effect on shrinkage temperature of collagen (Williams et al. 1995). There appears to be a difference between damage from short-term low-temperature exposure and long-term cold storage. |