EVALUATION OF CLEANING METHODS FOR THE EXTERIOR BRICK AT THE BROOKLYN HISTORICAL SOCIETYCLAUDIA KAVENAGH, & GEORGE WHEELER
ABSTRACT—The Brooklyn Historical Society's headquarters, designed by George B. Post, was constructed in 1881. This ornate brick and terracotta building is a landmark in downtown Brooklyn, New York. The building has never been cleaned or repointed. Heavy accumulations of black particulate soiling are present on both brick and terracotta. Many mortar joints are either failing or are completely without mortar. Recently, the Historical Society decided to develop a program for cleaning and repointing the exterior masonry. The project-specific difficulties in cleaning the brick can be attributed to the highly absorptive nature of the brick and the unusually high percentage of sulfates (caused by the use of a gypsum-gauged mortar in the original construction) within the brick. A sequence of site and laboratory analyses was performed to determine an appropriate method of cleaning that would not cause damage to the brick and would not increase the risk of future damage to the brick from salt migration. TITRE—�valuation des m�thodes de nettoyage pour la brique sur la face ext�rieure de l'�difice de la soci�t� historique de Brooklyn. R�SUM�—Le si�ge social de la soci�t� historique de Brooklyn a �t� con�u par George B. Post et construit en 1881. Ce b�timent tr�s orn�, qui est un des attraits du centre-ville de Brooklyn dans l'�tat de New York, est construit de briques et de terre cuite. L'ext�rieur du b�timent n'avait jamais �t� nettoy� et les briques n'avaient jamais �t� rejointoy�es. Des accumulations importantes de salet� noire sont pr�sentes sur la brique et la terre cuite. Le mortier se d�sint�gre dans plusieurs des joints et dans d'autres endroits, le mortier est m�me compl�tement disparu. R�cemment, la soci�t� historique a d�cid� de mettre en place un programme visant � nettoyer la ma�onnerie ext�rieure et � r�parer le mortier. Le nettoyage est rendu plus difficile � cause de la nature fortement absorbante de la brique et de son pourcentage exceptionnellement �lev� de sulfates (caus� par l'utilisation d'un mortier b�tard modifi� avec du gypse lors de la construction originale). Une s�rie d'analyses � l'emplacement m�me et en laboratoire a �t� compl�t�e afin de d�terminer une m�thode de nettoyage qui n'endommagerait pas la brique et n'augmenterait pas les risques de dommages caus�s par la migration de sels � l'int�rieur de la brique. TITULO—Evaluaci�n de m�todos de limpieza para el exterior de ladrillo en la Sociedad Hist�rica de Brooklynn. RESUMEN—La oficina principal de la Sociedad Hist�rica de Brooklyn, dise�ada por George B. Post, fue construida en 1881. Este ornamentado edificio de ladrillo y terracota es sitio destacado en el centro de Brooklyn, Nueva York. El edificio nunca hab�a sido limpiado ni la mamposter�a rejuntada anteriormente. Hay una gran acumulaci�n de part�culas negras en las superficies de ladrillo y terracota. Muchas juntas de mortero est�n sueltas y a punto de caerse o ya se cayeron. Recientemente, la Sociedad Hist�rica decidi� desarrollar un programa para limpiar y rejuntar la mamposter�a exterior. Las dificultades espec�ficas que este proyecto de limpieza presenta pueden atribuirse a la naturaleza altamente absorbente del ladrillo y el inusual alto porcentaje de sulfatos (producidos por morteros adicionados con yeso de la construcci�n original) dentro del ladrillo. Una secuencia de an�lisis de laboratorio y en el sitio se realiz� para determinar un m�todo apropiado de limpieza que no causara da�o al ladrillo y que no aumentara el riesgo de un futuro da�o del ladrillo por la migraci�n de sales. 1 INTRODUCTIONThe Brooklyn Historical Society is a nationally renowned urban history center dedicated to the exploration and preservation of documents, artwork, and artifacts representative of Brooklyn's diverse cultures. It contains a premier collection of research materials, including a wide variety of written materials, graphic images, and recordings of oral histories. The society's headquarters in Brooklyn Heights, New York, is an ornate brick and terracotta building As part of a larger campaign to rehabilitate the entire building, the historical society retained a series of consultants to develop and execute a testing program for cleaning and repointing the building.1 This article describes the testing program used to select materials and procedures for cleaning the exterior brick.
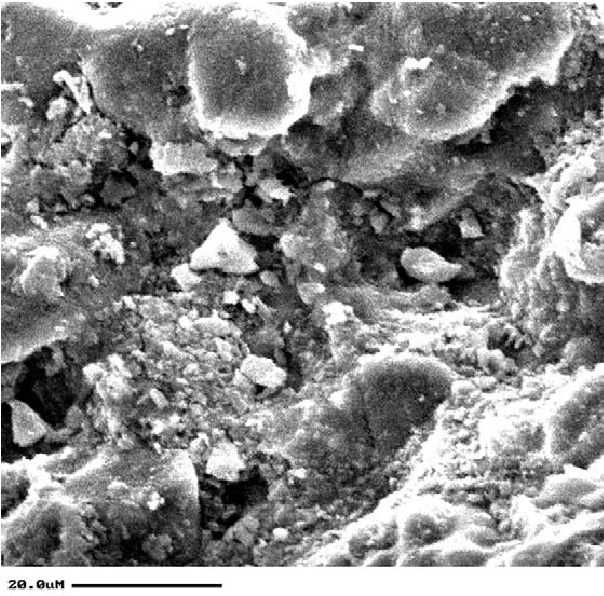
2 EXISTING CONDITIONS AND MATERIALS CHARACTERIZATION2.1 BRICKThe primary facades of the Brooklyn Historical Society are constructed of orange-red machine-pressed brick with smooth surfaces. Overall, the facades are in good condition except for areas that have been subjected to a great deal of water infiltration. The brick from the Brooklyn Historical Society may have been inadequately fired. Brick fired at high temperatures is red; the brick at the Brooklyn Historical Society is orange. Unlike adequately fired brick, the Brooklyn Historical Society brick is easily scratched and is highly absorptive (see absorption testing below) (Robinson 1982). Funds were not available to confirm this theory, nor was such a study necessary to achieve the project goal of the selection of a cleaning process for the brick. 2.2 MORTARMany of the mortar joints on the building are either failing or are completely missing (fig. 2). Mortar joint size varies but typically is narrow, in the range of 1/8 in. (3 mm) wide. The pointing mortar is typically gray and very fine in texture. The setting or bedding mortar is light tan and with a fine but visible texture. Both the gray and the tan mortars were analyzed by x-ray diffraction (XRD) and x-ray fluorescence (XRF). X-ray diffraction demonstrated that the gray pointing mortar contained gypsum and calcite; x-ray fluorescence indicated an average of 92% (atomic %) calcium and 8% sulfur for 20 measurements. No other elements were detected. Assuming all the sulfur in the mortar derived from gypsum, these percentages would correspond to approximately 13 parts lime and 1 part plaster of paris by volume in the original pointing mixture (the form of calcium sulfate added when the mortar was prepared is not known). If calcium carbonate were also used as aggregate, then the amount of lime in the original pointing mixture would be correspondingly reduced. XRD determined that the bedding mortar contained 2.3 SOILINGThe soiling on the brick consists of a thin but tenacious layer and follows a fairly regular pattern. Overall, there is light to moderate soiling on the majority of surfaces, with heavier buildup at the corners of the building. The soiling pattern on a typical individual brick face is roughly elliptical and occurs approximately 6–12 mm in from the brick edges. Closer examination reveals that there are white deposits and roughened surfaces associated with these soiling rings (fig. 3, see page 71). Samples of soiling were removed for analysis by scanning electron microscopy–energy dispersive spectroscopy (SEM-EDS). Scanning electron images of soiled and unsoiled brick are seen in figures 4 and 5. SEM-EDS found elements typical of flyash (silicon, aluminum, iron) as well as gypsum (calcium and sulfur) (Matero and Bede 1992). The presence of gypsum was also confirmed by Fourier transform infrared reflectometry (FTIR) and XRD.
3 CLEANING3.1 GENERAL CONSIDERATIONSBased on the degree of soiling, it appears that the Brooklyn Historical Society building has never been cleaned. Heavy accumulations (described in the previous section) are present on both the brick and the terracotta. The soiling is aesthetically disfiguring in that it largely conceals the intricate beauty of this ornamented building (fig. 6, see page 72). The soiling also clogs the brick's pores and contains hydrocarbons that limit the penetration of water through the exterior faces of the brick. While this condition might have a protective effect similar to an applied water repellent, it also moves the drying front for the brick from its external surface to some point below the surface. Moving the drying front below the surface, in combination with gypsum-bearing mortars and absorptive brick, can promote damage to the brick (fig. 7). During rain events, calcium sulfate is drawn into the brick. In periods of drying, salts crystallize below the surface rather than appearing as efflorescences on the surface. Therefore, cleaning of the building may be considered both for aesthetic reasons and to increase the durability of the brick.
It was decided early on in the project to test only commercially available cleaning materials. This decision was due to the scale of the project and the difficulties often encountered in obtaining uniform compositions of nonproprietary cleaning materials when complex on-site mixing is required. With that premise in mind, cleaners that might be effective in removing soiling were examined. Use of water alone was discounted because water is not a primary factor in the removal of black soil from brick, as the soil tends to be tenaciously adhered to the silicates in the brick (Ashurst 1994). Cleaning methods for brick fall into two categories: abrasive and chemical. Due to the observed softness of the brick surface, abrasive methods were not tested. Commercially available chemical cleaners for brick include detergents, acids, and bases and acids applied in sequence (Ashurst 1994). Detergents, while not damaging to brick, are also generally ineffective in removing black soiling
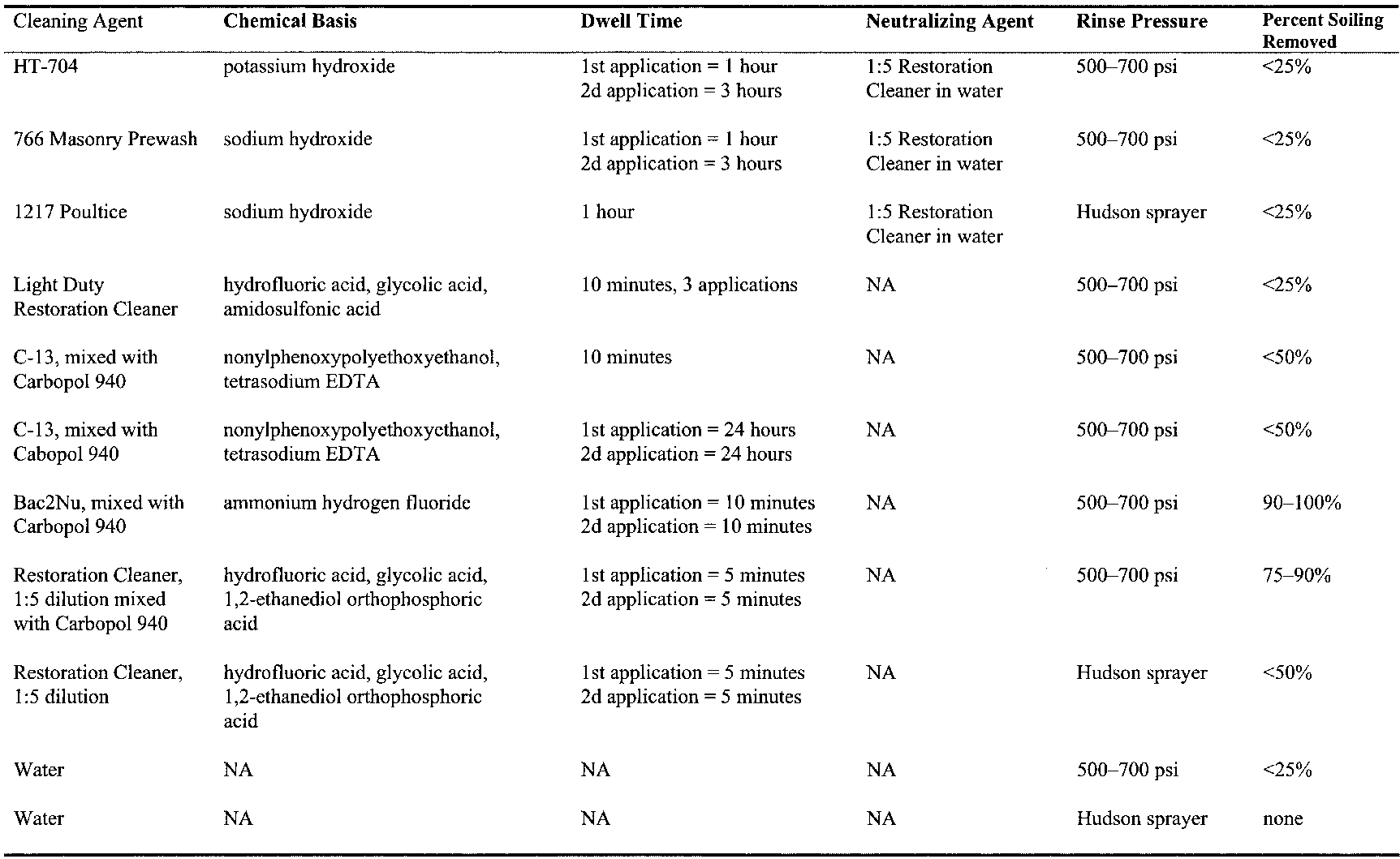
All cleaning methods involve risk. Strong bases and hydrofluoric acid are known to dissolve silicates (Iler 1979; Ashurst and Dimes 1990; Moynehan et al. 1995). Their use in cleaning brick requires determining the dwell times and concentrations required to expeditiously remove the soiling with minimal effect on the substrate. Alkaline cleaners containing sodium hydroxide carry the additional risk of producing sodium sulfate salts if the cleaner is not fully removed from the brick (Ashurst 1994). Another consideration for cleaning this specific building is to minimize the mobilization of gypsum in the mortars and, to a lesser degree, in the brick. Considering the large volumes of water employed in many cleaning processes, the risk of drawing additional gypsum from the mortar into the brick is not an unreasonable concern. 3.2 CLEANING METHODSAn initial set of cleaning tests was performed on discrete areas of brick surface that could not be seen from any public vantage point. The initial tests were performed to obtain an understanding of what types of commercially available chemical formulations would remove the soil from the brick. Tests were performed on brick exhibiting a wide range of soiling conditions, from light to very heavy, and included detergents, acids, and bases and acids applied in sequence. Since the brick absorbs liquid so rapidly, chemical cleaners of low viscosity were formulated as gels, using either methyl cellulose or Carbopol 940 to keep them at the surface of the brick during the prescribed dwell time (table 1). (At
The typical procedure was as follows:
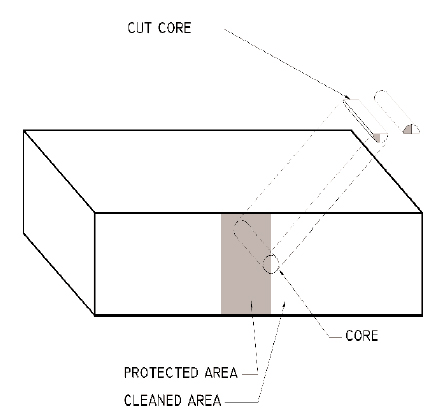
4 ASSESSMENT OF CLEANING METHODSOnly two products resulted in the removal of significant amounts of soiling: Bac2Nu, an ammonium bifluoride–based cleaner, and Restoration Cleaner, a hydrofluoric acid–based cleaner. After drying, a light white haze was observed on portions of the surface of the brick cleaned with Bac2Nu; it is likely that the chemical cleaner attacked the silica component of the brick and redeposited some of it onto the surface of the brick (Matero and Bede 1992, 37). The brick cleaned with Restoration Cleaner more closely matched the color of the brick face that had not been exposed to the weather and had no white haze. Further evaluations were performed to determine the effect of the cleaning methods on the brick. 4.1 EXAMINATION OF CROSS SECTIONSTo evaluate the effects of the cleaning materials and methods on the surface of the brick, the center third of each of three bricks was coated so that these areas would not be affected by cleaning2 (Mossotti et al. 2002) (fig. 8). The exposed surfaces of the three bricks were then cleaned using the following three methods: Brick One Restoration Cleaner, diluted 1:5 in water and mixed with Carbopol 940. Dwell time of 5 minutes. Two applications.
Brick Two Bac2Nu, mixed with Carbopol 940. Dwell time of 10 minutes. Two applications.
Brick Three
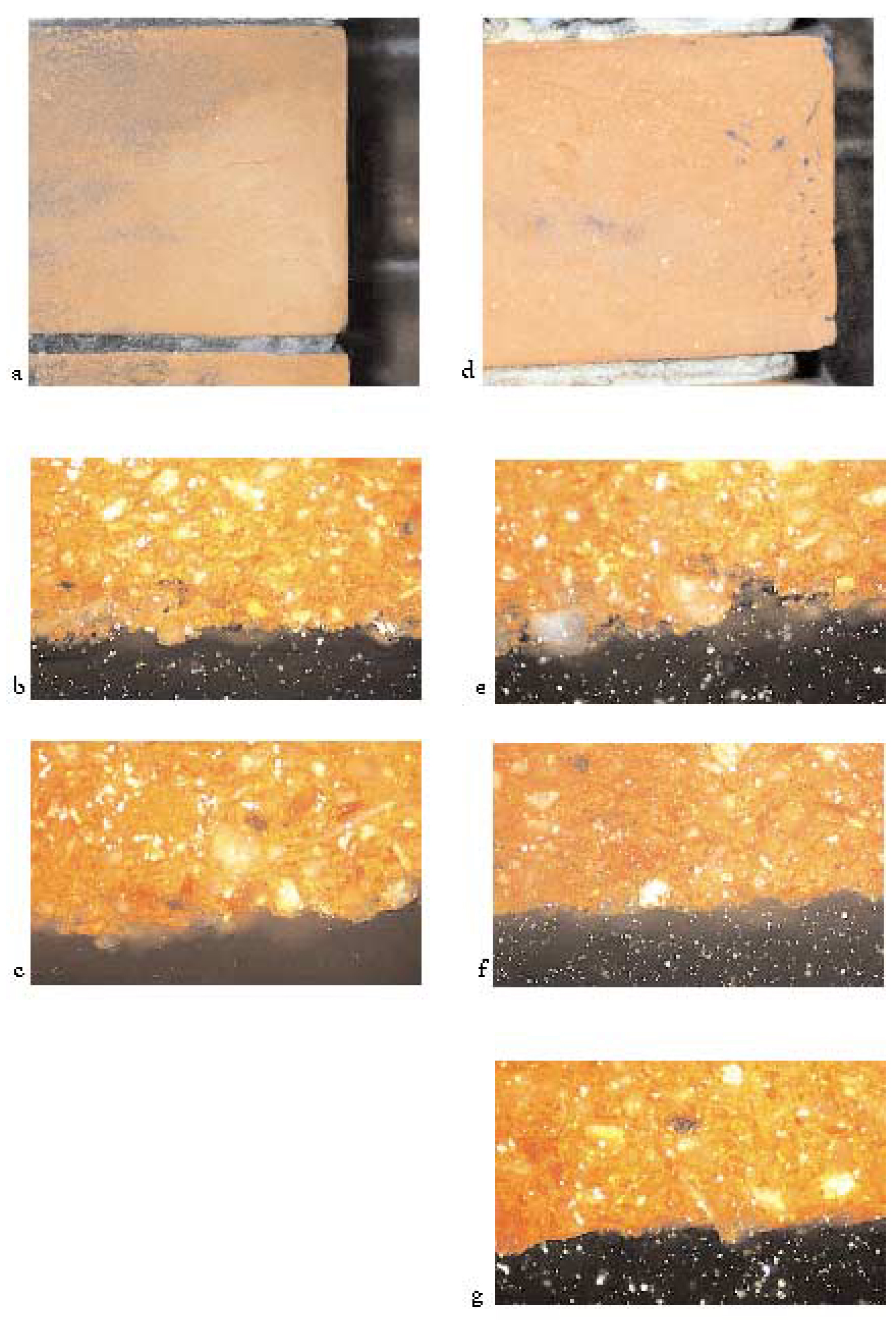
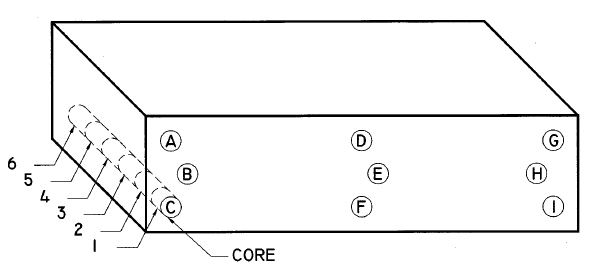
At the completion of the cleaning tests, the bricks were removed from the wall, and a series of 3/4 in. diameter cores were extracted. Each core was centered over the dividing line between the cleaned area and the protected area, so that one-half of each core was cleaned and one-half of each core was uncleaned. Each core was then split lengthwise so that the surface of the brick could be viewed in cross section, thus allowing for a direct comparison of cleaned and uncleaned surfaces (fig. 9). In addition, a core was removed from the back face of one brick to represent an unweathered, unsoiled, and uncleaned surface of the brick. Samples were viewed in reflected light at 25x magnification, using a Zeiss Axioplan II microscope. As can be seen in figure 10 (see page 73), the back of the brick (g) has a somewhat uneven and slightly rough surface with few openings or crevices observed. The uncleaned surface of each brick face that had been exposed to the weather was much
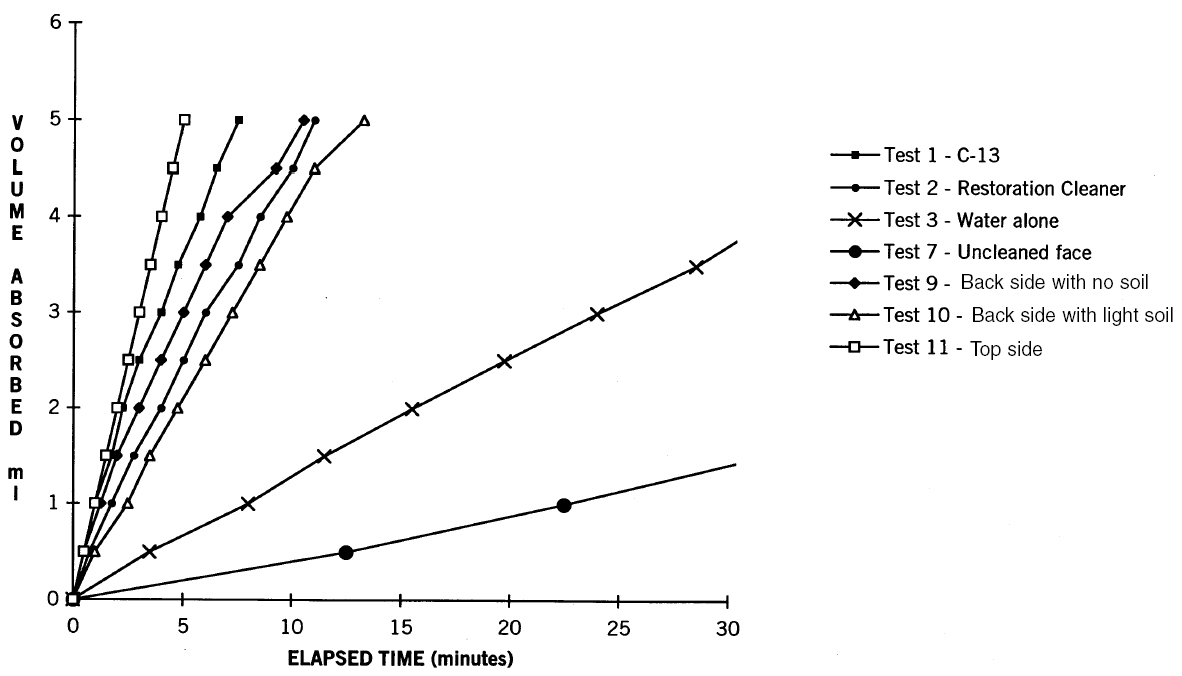
4.2 ABSORPTION TESTINGTests were performed on the brick to determine changes in this property brought about by different cleaning methods. Face brick were removed from the wall to perform the tests. The brick had little to no mortar adhering to the inner surfaces. Mortar remnants were soft and were easily removed from the surfaces. 4.2.1 RILEM Test Method No. II. 4R�union Internationale des Laboratoires d'Essais et de Recherche sur les Mat�riaux et les Constructions (RILEM) Test Method No. II. 4 (Gale 1989) (commonly referred to as the pipe method) was performed on soiled, weathered faces of brick, on the top side of bricks, on the back side of bricks (to simulate as closely as possible the condition of the brick when it was initially installed in the wall), and on representative cleaned surfaces. The RILEM test is a comparative method that measures the rate at which water is absorbed through the surface of a masonry element. A high-quality modern-face brick, graded SW (severe weathering) by ASTM standard C62 (ASTM 1996) to withstand harsh winters, will absorb little to no water over an extended period of time. On one uncleaned brick surface, 5 ml of water was absorbed in 26 minutes and 30 seconds, and on another test brick, the test was halted after one hour and 46 minutes when only 4 ml of water had been absorbed. In comparison, tests on the back side of 4.2.2 ASTM C67To further evaluate the effect of the cleaning methods on the absorption properties of the brick, the American Society for Testing and Materials (ASTM 1996) C67 Initial Rate of Absorption test was performed.3 The tests were performed on samples removed from the cleaned brick, from the back side of the same brick, and from the two different modern bricks. The rate of uptake of water for the brick cleaned with water alone was similar to but somewhat higher than for the back side of the same brick and similar to the two ASTM-standard bricks. Most important, uptake rates for chemically cleaned brick closely resembled those for the unweathered back sides of the same brick (fig. 12).
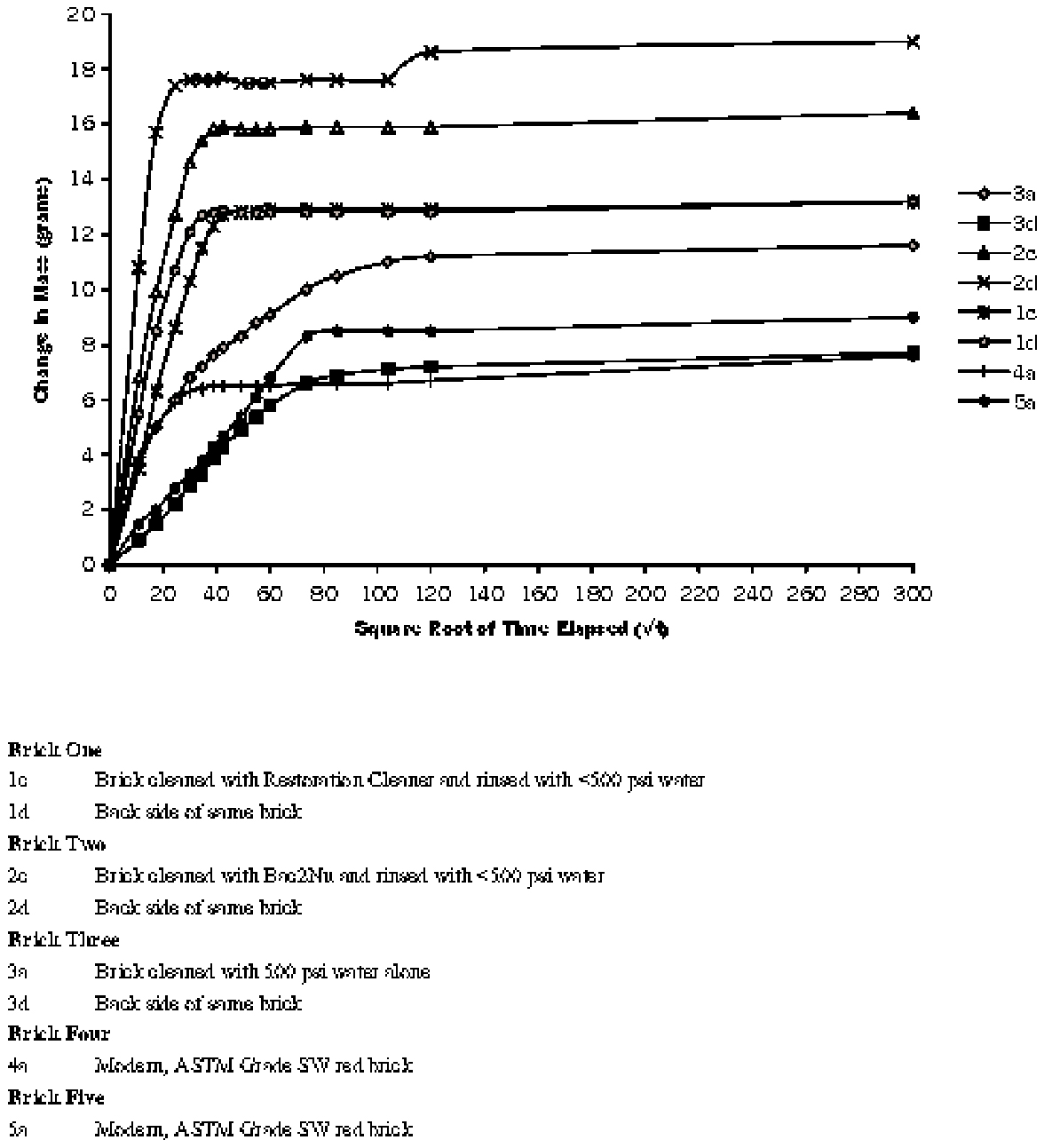
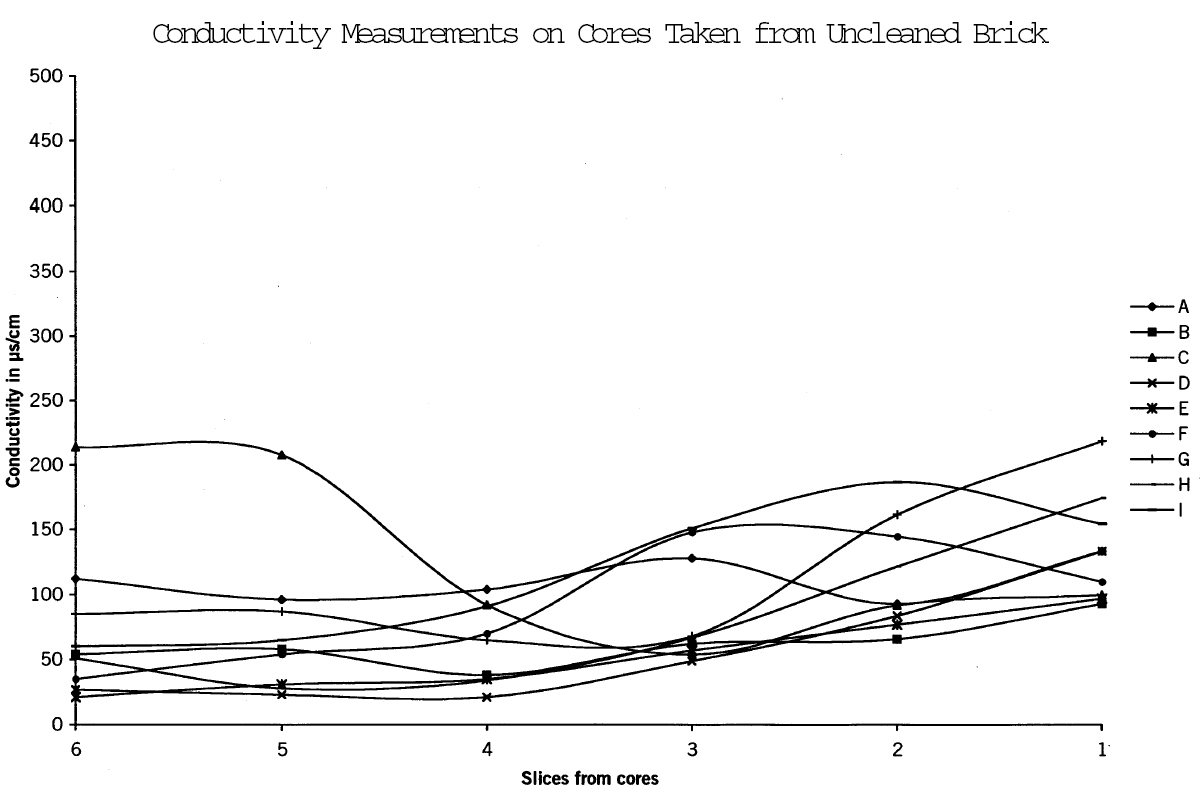
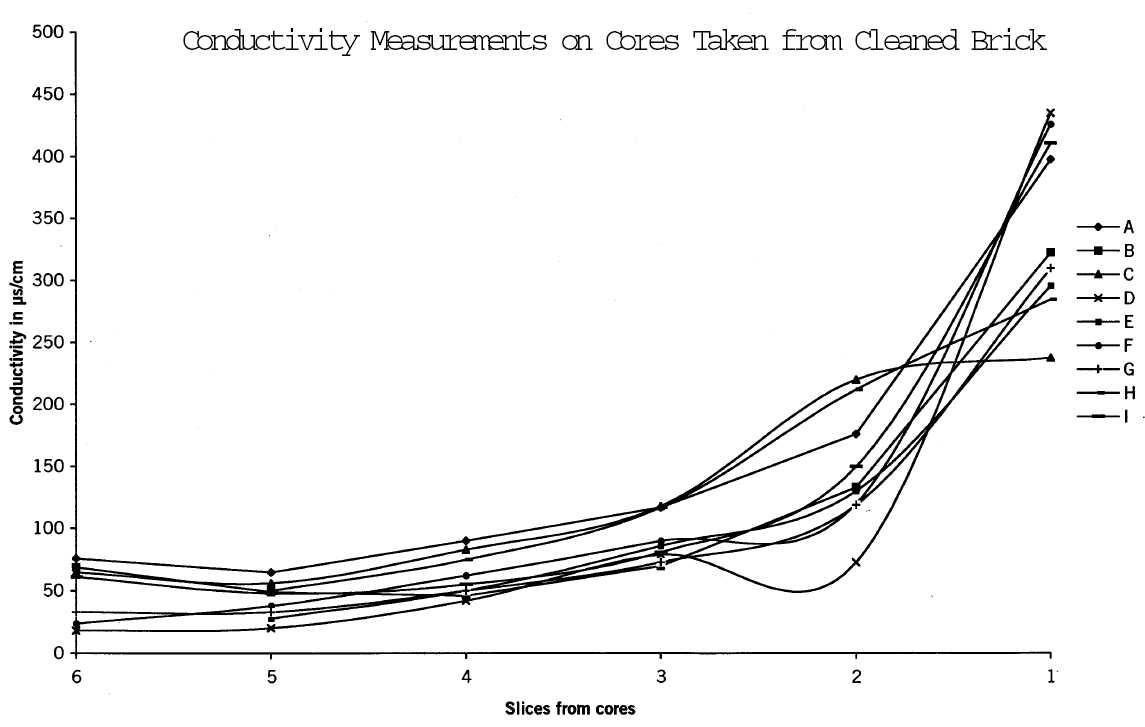
4.3 MOBILIZATION OF GYPSUMTo determine the amount and distribution of gypsum present in the brick before cleaning procedures were carried out and to ascertain whether or not the cleaning process mobilized gypsum, full-size bricks were removed from several locations on the building, including one that had been cleaned using Restoration Cleaner. Cores were removed from the bricks and sliced into sections. Each slice was weighed, then immersed in a measured amount of distilled water to produce a constant weight/weight ratio of brick to water. Conductivity measurements to determine salt content were made using an Accumet 50 pH/conductivity meter. Cores removed from the cleaned brick had significantly higher concentrations of salts at the surface (figs. 13–15) Microchemical tests also confirmed that these salts contained calcium and sulfate ions.
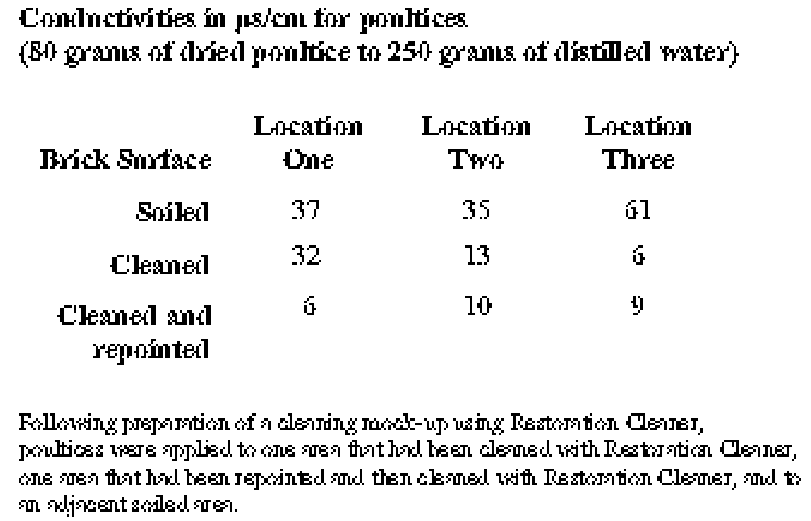
To determine if salts could be identified and subsequently removed by in situ poulticing, small-scale poultice tests were carried out on-site. For comparison purposes, poultices were applied at three locations each for soiled brick, brick cleaned with Restoration Cleaner, and brick that had been repointed and then cleaned with Restoration Cleaner. At each of the nine test locations, sterile cotton batting was submerged in distilled water and then placed firmly over an area approximating the size of one brick. The batting was covered with polyethylene sheeting, which was taped at the edges to retard evaporation. The batting was removed after 24 hours and brought to the laboratory, where conductivity measurements were made as described above. At soiled brick locations, conductivity readings ranged from 37 μs/cm to 61 μs/cm. At cleaned (but not repointed) brick locations, readings ranged from a high of 32 μs/cm to a low of 6 μs/cm. Significantly, at locations that had been repointed and then cleaned, readings ranged from only 10 μs/cm to 6 μs/cm (table 2). 5 DISCUSSIONThe Brooklyn Historical Society's building possesses two inherent “vices” that have contributed to its current condition: highly absorptive brick and mortars, both setting and pointing, which contain gypsum as a result of their original formulation. Because of these conditions, during a rain event, gypsum in the mortar is dissolved and the calcium sulfate solution is drawn into the highly absorptive brick. During drying, the solution is carried toward the surface of the brick, where gypsum reforms. The fact that the deposits follow the perimeter of the brick face supports this finding. In fact, the damage to the brick is particularly severe in those parts of the building where water management systems, such as gutters and leaders, have not been maintained. The building has also acquired the “vice” of soiling. This soiling disrupts the intended appearance of the building's surfaces and may also contribute to the deterioration of the brick. Particulates in the soiling block pores, and hydrocarbons in the soiling make the brick surfaces somewhat hydrophobic. These conditions are confirmed by the low capillary water uptake of soiled bricks in comparison to unweathered and unsoiled brick surfaces. These soiled surfaces can trap solutions of calcium sulfate inside the brick. Upon drying, gypsum crystallizes and damages the brick. For these reasons—both aesthetic and materials science—the cleaning of the building was entertained and a testing program carried out.
Due to the scale of the project and the difficulties often encountered in obtaining uniform compositions of nonproprietary cleaning materials when complex on-site mixing is required, only proprietary cleaners were tested. Of these, only Bac2Nu, an ammonium bifluoride–based cleaner, and Restoration Cleaner, a hydrofluoric acid–based cleaner, were effective in removing soiling from the brick. The Bac2Nu-cleaned brick exhibited a somewhat disfiguring white haze on the surfaces. Absorption testing demonstrated that the brick is indeed highly absorptive and that brick cleaned with Bac2Nu and Restoration Cleaner have an absorption rate similar to unsoiled and unweathered brick. Cross sections of cleaned and uncleaned brick clearly demonstrated that brick surfaces are smoother after cleaning with the HF-based Restoration Cleaner. Although the smoother surface was achieved because of small losses of material from the bricks, comparison of cross sections of brick cleaned with Restoration Cleaner with unweathered and unsoiled brick surfaces suggests that cleaning with the HF-based product results in a surface similar to the original surface of the brick. This smoother surface could result in a lower rate of resoiling and, therefore, less frequent cleaning.
Finally, there is the influence of cleaning on the gypsum-containing mortars. Conductivity testing showed that, unless the brick is repointed first, cleaning concentrates more gypsum at surfaces of the brick, and, therefore, increases the potential for damage to the brick. To reduce this risk, it was decided to remove existing pointing and to repoint the entire building with a lime-cement-sand–based mortar prior to cleaning. This repointing achieves two other desirable goals: (1) gaps left by missing pointing are now filled, thereby eliminating water infiltration during cleaning; (2) gypsum in the original pointing mortar is now removed, and the risk of future damage to the brick is reduced. 6 CONCLUSIONSThe cleaning of buildings is propelled by both aesthetic and materials science considerations. In the case of the Brooklyn Historical Society, both apply: (1) soiling has altered the play of light on ornamented surfaces of the brick and terracotta and has disrupted coloristic effects by random distribution of gray and black on otherwise orange-red surfaces; (2) particulate components of soiling block pores, and hydrocarbon components add water repellency to brick surfaces. While these conditions reduce infiltration of rainwater through the external surfaces and provide Several factors influenced the selection of cleaning materials and methods for the brick. First, the decision to use proprietary cleaners to ensure uniformity of composition narrowed the selection field. Second, the field was further narrowed in that only two of these proprietary cleaners were effective in removing soiling. One of these cleaners—the ammonium bifluoride–based Bac2Nu—left a disfiguring white haze, and the other, the hydrofluoric The final element in developing the cleaning process for the building was the decision to remove the gypsum-based pointing and replace it with a lime-cement-sand–based mortar before cleaning commenced. This method reduces the risk of movement of gypsum from the mortar into the brick and reduces subsequent damage during the cleaning process and for the future of the building. Project specifications included the testing of brick after cleaning to determine if salts were mobilized despite the precautions taken, with provision for their removal by poulticing if necessary. Masonry cleaning and repointing were performed during the spring, summer, and fall of 2002. At the completion of the exterior cleaning, a large percentage of soil had been removed from the brick, and the color of the brick was uniform and identical in color to the unweathered surfaces of the brick. Soil that was not removed after three applications of the specified cleaner was left on the brick (fig. 16, see page 74).
NOTES1. The first study was prepared by the Architectural Conservation Laboratory at the University of Pennsylvania in 1991–92. This research included a building history, terracotta characterization, mortar analysis, mock-ups using proposed repointing techniques, and limited laboratory and site cleaning tests. Integrated Conservation Resources Inc., a private firm with offices in New York City, undertook the second study, which concentrated on further exploration of cleaning methods. 2. A barrier coat of nitrocellulose glue was followed by a thicker coat of five-minute epoxy. The barrier coat was applied to prevent the migration of the five-minute epoxy, which is known to contain sulfur, into the brick. 3. In order to limit the test to the evaluation of the performance of only the exposed faces of the bricks, the sides of each sample were coated with Akemi Knife-Grade Buff. This coating formed a barrier to water so that uptake could occur only through the face of the brick. Cores measuring 82 mm in diameter were dried, weighed, and then partially submerged in water. The amount of water absorbed was measured by weighing the brick at set intervals. For comparison purposes, samples from five bricks were tested. ACKNOWLEDGEMENTSThe authors wish to acknowledge the contributions of previous testing performed by the Architectural Conservation Laboratory and by Integrated Conservation Resources Inc., as well as project support provided by the Brooklyn Historical Society. Thanks to Richard Pieper and Dean Koga for their insights throughout the course of the project. Scanning electron microscopy was performed by James Martin of Orion Analytical. Graphic contributions were provided by Danius Glinskis, Annie Vatterott, and Beth Romizer of Building Conservation Associates Inc. On-site testing was performed by Richard Pounds of ArchaTechnology Ltd. APPENDIXAPPENDIX 1 INSTRUMENTAL ANALYSESSEM-EDS generally was made using a Cambridge Stereoscan 100 scanning electron microscope coupled to a Tracor/Northern energy-dispersive spectrometer. Sample material generally was adhered to a carbon planchet or aluminum stub using conductive carbon tape, then coated with carbon or gold for analysis. X-ray diffraction was performed using a Philips 1710 open architecture diffractometer. Samples were ground to fine powder in an agate mortar and pestle and adhered to a quartz plate. Instrument parameters are: 40 kV and 30 mA of copper radiation, 5–65 degrees of Bragg angle. Diffractograms are processed X-ray fluorescence was performed on a Jordan Valley 3600 spectrometer. Analyses were performed nondestructively (i.e., without sample preparation) using 10 kV radiation from a rhodium tube and amperage to produce a dead time between 40% and 50%. For FTIR, a Spectra-Tech IR Plan Research microscope was coupled to a Nicolet Magna 550 FTIR spectrometer. Both instruments were purged with dry air. Sample material generally was compressed onto a single Spectra-Tech diamond cell for analysis by transmission at 4 cm–1 resolution for 32–132 sample and background scans using Happs-Genzel apodization. REFERENCESAshurst, J., and F. G.Dimes. 1990. Conservation of building and decorative stone, vol. 2.London: Butterworth-Heinemann. Ashurst, N.1994. Cleaning historic buildings. Vol. 1, Substrates, soiling and investigations. Vol. 2. Cleaning materials and processes. London: Donhead Publishing. ASTM. 1996. Standard specification for building brick (solid masonry units made from clay or shale), C62-95a, and standard test methods for sampling and testing brick and structural clay tile, C67-94. West Conshohocken, Pa.: American Society for Testing and Materials. Boynton, R. S.1980. Chemistry and technology of lime and limestone. New York: John Wiley. Gale, F.1989. Measurement of water absorption. APT (Association for Preservation Technology International) Bulletin21(3 & 4):8–9. Historic Scotland. 1992. Stone cleaning in Scotland: Research summary. Glasgow: Historic Scotland. Hodgson, F. T.1916. Mortars, plasters, and stuccos. Chicago: Frederick J. Drake. Iler, R.1979. The chemistry of silica. New York: John Wiley. Matero, F. G., and E.Bede. 1992. Characterization and assessment of cleaning and grouting techniques for unglazed terracotta and brick: The Brooklyn Historical Society. Unpublished typescript. Architectural Conservation Laboratory, University of Pennsylvania. Maxwell, I.1995. Preparation and use of lime mortars. Edinburgh: Historic Scotland. Mossotti, V. G., A. R.Eldeeb, T. L.Fries, M. J.Coombs, V. N.Naude, L.Soderberg, and G. S.Wheeler. 2002. The effect of selected cleaning techniques on Berkshire Lee marble: A scientific study at Philadelphia City Hall. Professional Paper 1635. CD-ROM. Washington, D.C.: U.S. Department of the Interior, U.S. Geological Survey. Moynehan, C. R., G. C.Allen, I. T.Brown, S. R.Church, J.Beavis, and J.Ashurst. 1995. Surface analysis of architectural terracotta including new and soiled examples, and pieces treated with a hydrofluoric acid-based cleaning solution. Journal of Architectural Conservation1(1):56–69. Robinson, G. C.1982. Characterization of bricks and their resistance to deterioration mechanisms. In Conservation of Historic Stone Buildings and Monuments. Washington, D.C.: National Academy Press. Willensky, E., and N.White. 1988. AIA guide to New York City. New York: Harcourt Brace Jovanovich. FURTHER READINGBuilding Conservation Associates Inc.2000. Brooklyn Historical Society: Brick and terracotta cleaning tests. Unpublished typescript. Building Conservation Associates Inc., New York. Integrated Conservation Resources Inc. 1998. Conservation services: The Brooklyn Historical Society. Unpublished typescript. Integrated Conservation Resources Inc., New York. SOURCES OF MATERIALSAkemi Knife-Grade BuffJaeger and Condino Inc. P.O. Box 592 35-44 61st St. Woodside, N.Y. 11377 (718) 335-8300 Bac2Nu Stone & Masonry Cleaner/RestorerChemique Inc. 315 N. Washington Ave. Moorestown, N.J. 08057 (800) 225-4161 Carbopol 940B. F. Goodrich Specialty Polymers and Chemical Division 9911 Brecksville Rd. Cleveland, Ohio 44141-3247 (800) 331-1144 Restoration CleanerPROSOCO Inc. 3741 Greenway Circle Lawrence, Kans. 66046 (800) 255-4255 AUTHOR INFORMATIONCLAUDIA KAVENAGH received her M.S. in historic preservation from Columbia University's Graduate School of Architecture, Preservation, and Planning. She is director of the New York offices of Building Conservation Associates Inc., where her work combines the disciplines of historic preservation and materials conservation for the restoration of buildings and monuments. Address: Building Conservation Associates Inc., 158 West 27th St., New York, N.Y. 10001 GEORGE WHEELER is research chemist at the Sherman Fairchild Center for Objects Conservation at the Metropolitan Museum of Art. He holds a master's degree in art history from Hunter College of the City University of New York, a certificate in conservation from New York University's Conservation Center, and a Ph.D. in chemistry, also from New York University. His main area of research is the conservation of stone. Address: Metropolitan Museum of Art, 1000 Fifth Ave., New York, N.Y. 10028
 Section Index Section Index |