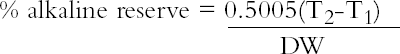EFFECTS OF ENCLOSURE PAPERS AND PAPERBOARDS CONTAINING LIGNINS ON PHOTOGRAPHIC IMAGE STABILITYDANIEL M. BURGE, JAMES M. REILLY, & DOUGLAS W. NISHIMURA
ABSTRACT—The photographic activity test (PAT) was used to quantify the effects of lignin-containing papers and boxboards on photographic image stability. For papers in direct contact with photographs, as lignin content increased, both gelatin staining and silver image interaction increased. Tnhese effects were somewhat mitigated by the inclusion of calcium carbonate buffering in the paper. For boxboards surrounding but not in direct contact with photographs, the effects were only slightly mitigated by the air space between lignin-containing board and test detectors. It was also determined that other wood components, collectively known as extractives, were also reactive with silver images. These extractives are, however, simultaneously removed with lignin during pulp delignification. TITRE—Effets des emballages en papier et en carton contenant de la lignine sur la stabilit� de l'image photographique. R�SUM�—Le test d'activit� photographique (PAT) a �t� employ� pour mesurer les effets qu'ont les papiers et les cartons contenant de la lignine sur la stabilit� de l'image photographique. Pour les papiers en contact direct avec des photographies, l'incidende des taches dans la g�latine et l'interaction avec l'image argentique se sont accrus � mesure que le contenu de lignine augmentait. Ces r�actions ont �t� l�g�rement att�nu�es par l'introduction dans le papier d'un tampon de carbonate de calcium. Pour les cartons d'emballage n'�tant pas en contact direct avec les photographies, les r�actions ont �t� seulement l�g�rement att�nu�es par l'espace entre le carton contenant de la lignine et les agents d�tecteurs utilis�s lors des tests. On a �galement d�termin� que d'autres composants du bois, g�n�riquement rassembl�s sous le terme extraits du bois, �taient �galement nocifs pour les images argentiques. Cependant, ces extraits sont simultan�ment enlev�s avec la lignine pendant la purification de la p�te � papier. TITULO—El efecto de las fundas de psapel y cartulina, que contienen lignina, en la estabilidad de la imagen fotografica. RESUMEN—La prueba de actividad fotogr�fica (photographic activity test–PAT) se utiliz� para cuantificar los efectos nocivos de papeles y cartulinas, que contienen lignina, en la estabilidad de la imagen fotogr�fica. Los papeles y cartulinas en contacto directo con las fotograf�as provocaron, a medida que su contenido de lignina aument�, mayor decoloraci�n de la capa de gelatina y m�s alteraci�n en la imagen de plata. Estos efectos disminuyeron, hasta cierto punto, al usar papeles tamponados (buffered) con carbonato de calcio. As� mismo, el efecto nocivo de las cartulinas de cajas que protegen a las fotograf�as, pero sin entrar en contacto con ellas, fue menor debido al espacio de aire entre las cartulinas con lignina y los detectores de la prueba. Tambi�n se determino la reactividad de otros componentes de la madera, conocidos gen�ricamente como extractos, sobre la imagen de plata. No obstante, estos extractos se eliminan simult�neamente con la lignina durante la deslignificaci�n de la pulpa. 1 INTRODUCTIONPulps are the sources of cellulose fibers that make up paper and paperboard. There are two primary sources for pulps: cotton and wood. Historically, cotton has been considered the more stable of the two pulps and, therefore, by default, the most appropriate for use in contact with photographic images. Unfortunately, papers and paperboards made from cotton pulps can be very expensive. The alternative, wood pulp, is much cheaper. Historically, wood pulp developed a bad reputation as a result of negative experiences with papers made from nonpurified wood pulps. These woodpulp papers quickly became acidic, discolored, and brittle. The degradation was attributed to lignin, the primary noncellulose component of the wood, as well as to the acidic alum-rosin sizes used. Similarly, lignin-containing envelopes and boxes used to house photographs have been blamed for the degradation of enclosed images. As a result of the concern over lignin in wood It has never been shown experimentally, however, that lignin is a cause of photo degradation (fading, mirroring, red spots, or staining). It is the purpose of this study to examine the effects of lignincontaining paper and paperboards on photo stability. There are a large number of pulping processes. The simplest involves mechanically grinding logs to separate the fibers. The resulting product is referred to as groundwood. The pulping process can be speeded up by the application of heat, a process known as thermo-mechanical pulping. Both processes leave most of the lignin in the pulp. Pulping processes that remove or reduce the lignin use chemical reaction to dissolve the lignin. This process is referred to as digestion. Lignins vary in composition among tree species, and even among individual trees, and are more correctly referred to in the plural. Lignins are complex polymers that are amorphous and threedimensional in structure. They function chiefly as a filler or cement substance to impart rigidity to wood tissue. In North American woods, lignins content is approximately 18–25% for hardwoods and 25–35% for softwoods (Biermann 1996). They are difficult to study because there is no known method for isolating them that does not alter them chemically and quantitatively. Lignin molecules are extremely large, having molecular weights in the thousands, and their chemical structures can take innumerable forms. A concept important to the pulp and paper industry is the yield. Yield refers to the ratio of the weight of the wood going into a pulping process to the weight of the pulp produced. When lignins are removed, the yield is reduced and less paper can be produced. So, just as there is a price connection with pulp sources (cotton versus wood), there is a price connection to level of delignification. The more lignins that are removed, the smaller the yield and the greater the final paper cost. By-products from the chemical pulping processes are not considered waste. Other industrial products can be extracted from the pulping residuals, and whatever is left can be burned for production energy. However, these savings are not enough to offset the loss in pulp yield. Various methods are used to reduce lignins content during pulping. Some lignins can be removed in an acid environment and some in an alkaline environment. Both approaches have been used, although alkaline pulping is currently the more common. Not every pulping process fully removes lignins. There is a continuum ranging from highlignin pulps such as groundwood down to fully purified pulps such as kraft. In between are the semipulps. These pulps are produced through various combinations of mechanical grinding, heat, and chemical actions by which the cellulose fibers are freed from wood and lignins content is reduced. The advantage is that the papers produced from semipulps are somewhat stronger and longer lasting than those produced from groundwood pulps but still less expensive than those produced from fully purified pulps. Beyond the cellulose fibers and lignins, wood also contains small amounts of a variety of other compounds. These are referred to collectively as the extractives, because either organic solvents or water can extract them. Like lignins, the composition of extractives within wood varies from species to species and even within a given species, depending on growth conditions. The extractives can impart color, taste, and decay resistance to wood. In North American woods, the extractives content is approximately 1–5% for hardwoods and 3–8% for softwoods. There are also traces (less than 1%) of inorganic substances in wood (Biermann 1996), but they are not under consideration. In the paper industry there has been a movement to establish papers made from semipulps as appropriate for permanent documents. Experiments examining paper performance characteristics, such as tensile strength and fold endurance, have suggested that pH is the prime indicator of paper permanence, not lignins content (B�gin et al. 1998). The suggestion is that lower-cost semipulps could be used to make Buffering has been included in papers for use in photographic enclosures to absorb any acid from the environment, the photograph, or the enclosure itself. It has been shown that acids can attack photographic film supports (Adelstein et al. 1995) and paper supports and that some acids can directly oxidize silver images (Carroll and Calhoun 1955). Unfortunately, in an effort to put those all-important words “acid-free” on their packages, some producers have been adding buffers to paperboards made with acidic groundwood pulps. The result is that pH tests may show a high alkaline reading, while the board may, in fact, contain acids or potential acid sources. It is worth noting that pH cannot be used as an indirect method of determining lignins content. Acid-free does not equate to lignin-free. Finally, as manufacturers have striven to produce inert storage enclosures for use in direct contact with photographs, there has developed a question as to whether paper or paperboard products that do not come in direct contact with the photograph are required to be free of lignins as well. Many boxes, album covers, and slipcases are made with ligninscontaining cores covered by cloth on the exterior and paper liners on the interior. There is anecdotal evidence suggesting that lignins-containing boxes degrade photographic materials stored inside. However, no study has been completed to demonstrate the effect. Given the above discussion, the specific questions associated with this problem and addressed by this study are the following:
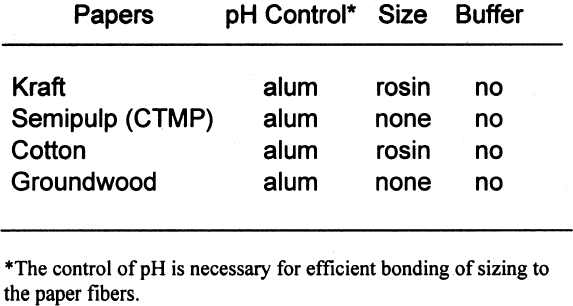
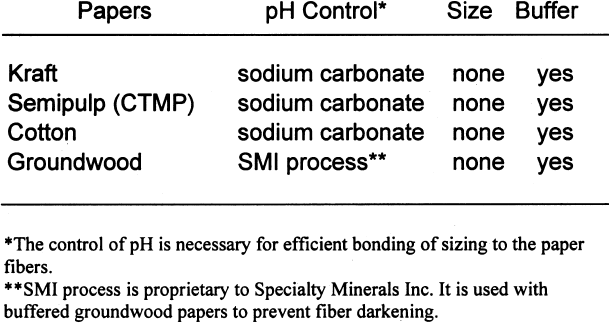
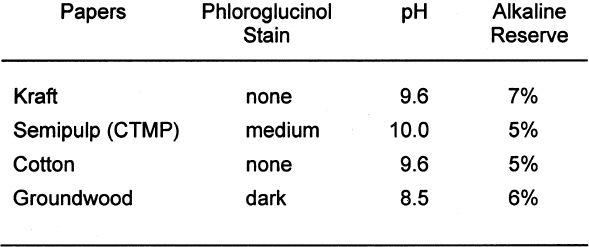
It should be noted that the results of this study apply to the storage of photographs only and not to the storage of other archived materials. This study also examines the effect of lignins on black-andwhite silver images only and not their glass, metal, paper, or plastic supports. 2 SAMPLE PREPARATION2.1 PAPERSIn the mid-1990s, a project conducted by the Institute for Standards Research of the American Society for Testing and Materials (ASTM) and sponsored by several paper companies produced papers of known composition in order to examine the effects of paper ingredients on paper longevity. The Image Permanence Institute (IPI) at the Rochester Institute of Technology was contracted to perform pollution exposure tests on the papers as part of the larger project. The availability of these special papers provided a rare opportunity for IPI to test the effects of paper ingredients on photographic materials. ASTM was gracious enough to provide a few sheets of each paper type for these tests. The overall design of the study was to characterize the paper samples using well-established analytical methods and then measure the effects on photographic materials. The majority of the tests performed for this project were done using a set of eight papers of controlled, varied composition. The variations were in pulp type, sizing, and buffering. The pulps used were cotton, bleached softwood kraft, bleached softwood chemical-thermal-mechanical (CTMP), and groundwood. Kraft pulps were fully purified to remove all measurable traces of lignins. The CTMP is a semipulp, so while most of the lignins were removed, some still remained. As mentioned above, groundwood pulp is produced by the mechanical grinding of logs. Most of the lignins present in the wood before grinding are present in the resulting pulp. Alum (aluminum sulfate)-rosin sizing was used in The first set of tests performed was a simple
The presence of lignins was determined by using the phloroglucinol spot test. The solution for the test consists of 1 g phloroglucinol in a mixture of 50 ml of water, 50 ml of methyl alcohol, and 50 ml of concentrated hydrochloric acid. Applying a small drop of the solution to the surface of the paper or board will turn any ligneous fiber dark purple. The depth of the color change in the test area is proportional to the amount of lignins present. For the pH test, approximately 1 g of the material was placed into a 250 ml flask. Seventy ml of distilled water with a pH of 5.9 or higher was added to the sample. The samples were then allowed to soak for one hour. The pH was then measured with a Beckman pHI 40 pH meter. Two replicates of each paper were tested, and the results were averaged. Note that the standard allows the distilled water to have a slightly acidic pH. This allowance is due to absorption of carbon dioxide (CO2) from the atmosphere. When samples of an acidic or alkaline nature are tested, this slight acidity of the water should have a negligible effect. However, it does become difficult to accurately measure samples having a pH close to that of the test water. The alkaline reserve test used here is a modified version of ASTM D 4988–96 determination of alkalinity of paper as calcium carbonate (alkaline reserve test of paper) (ASTM 1996). Approximately 1 g of the material was placed in a flask. Added to the flask was 70 ml of distilled water. The mixture was allowed to soak one hour with occasional agitation. After soaking, 25 ml of 0.1 N hydrochloric acid (HCL) was added. The samples were then gently boiled for one minute to expel carbon dioxide (CO2). After cooling back to room temperature, the samples were titrated with 0.1 N sodium hydroxide (NaOH) to change in bromothymol blue indicator from yellow to blue. Two blanks also were tested. The following equation was used to calculate percent alkaline reserve. Two replicates of each material were tested, and the results were averaged.
T2 is the NaOH required for the blank. T1 is the NaOH required for the sample. DW is the dry weight of the sample in grams.
Tables 3 and 4 show the results of the analysis of the paper samples. The results of the paper analysis were almost all as expected. The one variable that seemed above normal was the pH of the buffered papers. The higher-than-expected pH values are probably due to the use of sodium carbonate in the papermaking process to control pH. The pH test measures the total pH of the paper, not merely the calcium carbonate content. Therefore, contributions to overall pH will be the sum of the pulp, the pH control, and the buffer. The nonbuffered papers used alum as a pH control. 2.2 EXTRACTED PAPERSThe role of wood extractives was studied by removing them from paper samples before performing tests for inertness toward photographs. For the samples in which the extractives were removed from the papers, the extraction method described in TAPPI T204 om-88 solvent extractives of wood and pulp (TAPPI 1988) was used. This method employs a Soxhlet apparatus (fig. 1). A variety of solvent or solvent mixtures are suggested. For this experiment the ethanol-toluene mixture was selected. This solvent mixture has had poorer reproducibility than the ethanol-benzene mixture when determining the exact concentrations of extractives. However, since the goal of this experiment was simply the removal of the extractives and not quantification of the extractives, it was felt that the ethanol-toluene mixture would be more appropriate, as it posed the smallest health risk.
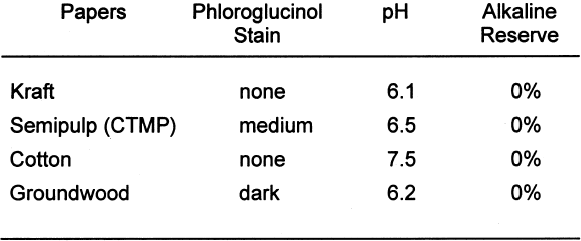
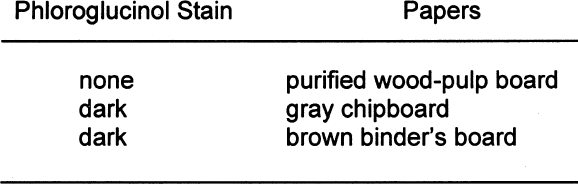
Twelve-by-two-cm strips of each of the nonbuffered paper types were extracted for eight hours in the extractor. The extracted samples were then dried on blotter paper. 2.3 BOXESThe samples for the box test were constructed at IPI. Three sets of boxes were constructed. One was from gray chipboard purchased from the Rochester Institute of Technology bookstore. One was from the core of a three-ring binder, and another was from a buffered, purified wood-pulp board to be used as the control. The purified wood-pulp board was Exeter Conservation Board purchased from Light Impressions The lids on the boxes fitted tightly except for the one on the gray chipboard box, which, due to a construction error, had a small gap of 2 mm along one side. The boxes were lined on the bottom with polyester to prevent direct contact between the test detectors (see PAT below) and the bottom of the box. The size of the boxes was 3 � 16.5 � 16.5 cm. No interior paper liners were used in the boxes, unlike most boxes that are not solid construction throughout. The boxboards were tested with the phloroglucinol spot test to verify lignins content. A dark stain indicates the presence of lignins. Table 5 shows the results of the phloroglucinol spot test on the boxboards.
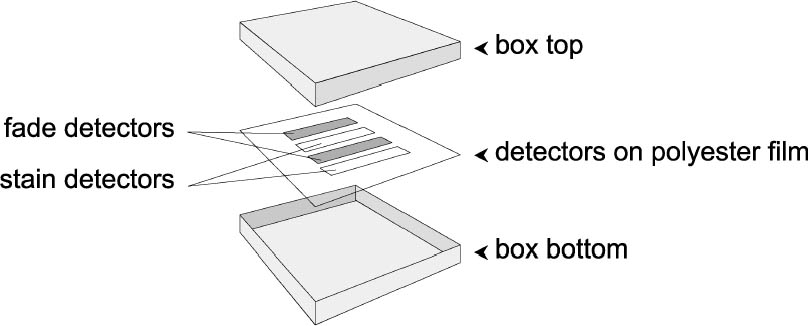
3 EXPERIMENTAL METHODS3.1 PHOTOGRAPHIC ACTIVITY TEST (PAT)The photographic activity test of ISO Standard 14523 (ISO 1999) was chosen for these experiments, as it is the sole method recognized by the International Organization for Standardization for predicting the long-term interactions between enclosures and photographs. Other test methods, such as the Oddy Test (Oddy 1973, 1975) or the Collings and Young silver tarnish test (Collings and Young 1976) may be able to screen paper and paperboards for negative reactions with metallic silver. However, those tests use silver plates as opposed to silver particles embedded in gelatin like the detector used in the PAT. Also, neither test examines the effects of materials on photographic gelatin. The PAT uses two detectors to simulate a photographic image. The first PAT detector, called the image interaction detector, consists of colloidal silver particles distributed through a gelatin coating on polyester film. This detector is used to measure oxidation and reduction reactions with silver. Such reactions cause fading, mirroring, and red spots in photographs. Fading is a loss in image density either overall or localized (localized fading is called mottling). Fading is the result of the oxidation of the metallic silver particles forming the image. The metallic particles are stripped of electrons and converted to silver ions. These ions are not visible, hence the loss in image density. Mirroring and red spots are similar to fading in that they involve oxidation, but they also involve migration of the silver ions and their subsequent reduction back to metallic silver in new locations. In mirroring, the silver ions are converted back to metallic silver at the surface of the print or film. This metallic silver forms a reflective sheen. In red spots, the silver ions are redeposited at sites seeded by embedded impurities such as metals, scratches in the gelatin, or image areas of great contrast. These deposits are reddish orange in color. The second PAT detector, called the gelatin stain detector, is a strip of white processed photographic paper. This detector is used to predict discolorations in the gelatin binder. Black-and-white photographic paper is used to avoid the complications of residual dye couplers found in chromogenic products. According to the standard, the papers being examined for photoactivity are stacked (fig. 2) with two each of the above detectors within a holding jig. Strips of Whatman no. 1 filter paper are used as interleaves between the paper samples and the image interaction detectors. Filter paper interleaves were not included in the gelatin stain tests, as it was not The PAT was used to test all the paper samples as well as the extracted versions of the paper samples.
3.2 BOX TESTThe box test used the same two detectors as the PAT. The method varied from the PAT in that the detectors were placed emulsion-up in the center of the bottom of the box. To prevent direct contact with the boxboard, the detectors were separated from the bottom of the box by a sheet of polyester (fig. 4). A box could not be made from Whatman no. 1 filter paper (the control used in the PAT), so the control box was made from the buffered, purified wood-pulp board and the same adhesive.
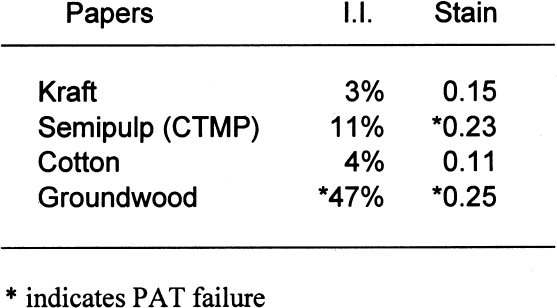
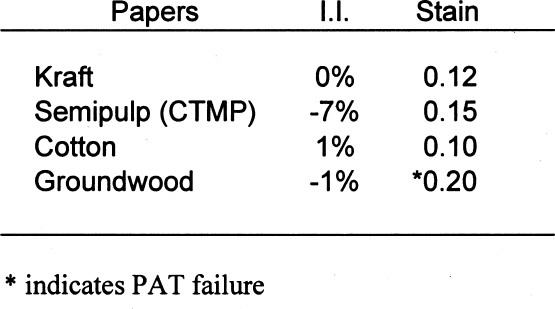
The PAT was also used to test the boxboard materials in direct contact in order to examine the dilution effects of the air space within the box. 4 RESULTS4.1 PAPERSTable 6 shows the results of the PAT on the four nonbuffered papers. Negative image interaction (I. I.) values indicate the percent difference that the sample detector faded more than the control (oxidation reactions). Positive image interaction values indicate the percent difference that the sample faded less than the control (oxidation and/or reduction reactions). The standard states that the pass/fail limits for the image interaction portion of the test will be � 20% of the control. Of the four papers, only the groundwood
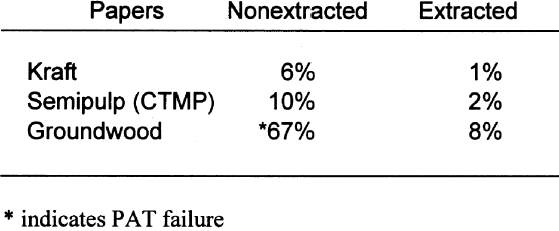
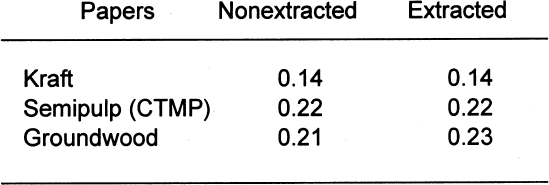
The standard states that the pass/fail limit for the stain portion of the test will be 0.08 density units above the control. In this case, the control result was 0.11; therefore, the stain limit is 0.19. Of the four papers, both the groundwood and the semipulp papers failed the gelatin stain test. This finding indicates that medium to high lignins content results in gelatin stain. Table 7 shows the results of the PAT on the four buffered papers. Again, the pass/fail limits for the image interaction portion of the test are �20% of the control. None of the four papers failed the image interaction test. It appears that the inclusion of buffers in papers containing lignins can mitigate the adverse effects of lignins on silver image particles. The pass/fail limit for the stain portion of the test is 0.08 density units above the control. In this case, the control result was 0.11; therefore, the stain limit is 0.19. Of the four papers, only the groundwood paper failed. This finding indicates that high lignins content may result in gelatin stain regardless of the inclusion of a buffer in the paper. 4.2 EXTRACTED PAPERSIt is not necessarily correct to assume that it is the lignins from wood pulps that cause all harmful reactions with photographs. There is still the question of the reactivity of the extractives. That these materials may be the harmful component of lignins-containing pulp may be moot. They seem to be removed in the delignification and bleaching processes. It is possible, though, that establishing these materials as potential sources of photoactivity may help papermakers produce lower-cost, quality papers in the future. Tables 8 and 9 show the results of those tests. The removal of extractives significantly changed the
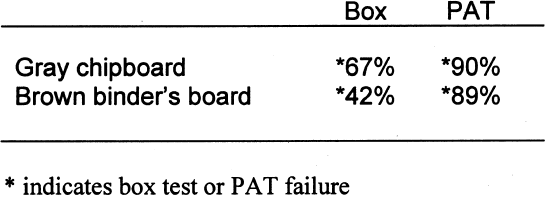
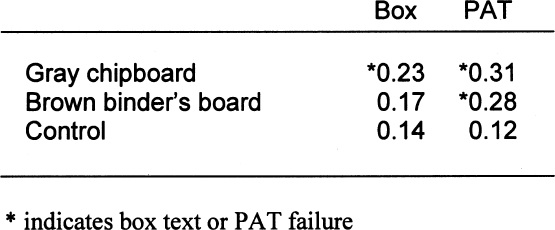
4.3 BOX TESTIn the above tests, the lignins-containing paper products were tested in intimate contact with the image interaction and gelatin stain detectors. This arrangement simulates the practice of housing images in direct contact with enclosures such as envelopes, sleeves, and mats. But what about products that do not come in contact with the images, such as slipcases, boxes, and album binders? Can harmful groundwood components in these materials act across the air spaces inside the box? The boxboards were tested in the box test and in the PAT simultaneously to compare results. Note that in the PAT configuration the box material is in direct contact with the detectors. In the box test configuration, the test detectors are separated from the box material by 3 cm. Because of this air space, some reduction of the signal was expected. Also note that the control for the PAT was Whatman no. 1 filter paper, and the control for the box test was made from buffered, purified wood-pulp mat board. The results are shown in tables 10 and 11. As expected, the boxes made from the gray chipboard and the brown binder's board failed the box test and the PAT in image interaction. However, the dilution of harmful contaminants in the box's air space did reduce the staining effect enough for the brown binder's board to pass the test. Still, it is clear from the results that lignins-containing paper products do not need to be in direct contact with the images in order to mar them. The density difference between the test detectors and the control test detectors was less for the box test than for the PAT. This finding is due to dilution of the harmful volatiles within the air space of the box. However, the harmful action still exists. 5 DISCUSSIONSFrom the PAT results on the various papers, it seems appropriate to recommend that nonbuffered papers It also appears from the PAT results that photoinert buffered paper enclosures can be made from semipulps as well as from cotton or fully delignified pulps. However, semipulps are not delignified to any standard or predictable value, and therefore actual lignins content will vary. Although these results indicate that some enclosures made from semipulps may be suitable for use in direct contact with photographs, great care should be exercised in their selection. Fully delignified papers are preferable because of reduced risk of gelatin staining. For the time being, the discussion of whether the harmful effects of lignins-containing papers come from lignins or the extractives is moot. The extractives are removed in direct proportion to lignins during pulping. Differentiating the reactivity of the two may become more important during further investigations of the photo-reactivity of semipulps. From the box test results, it appears that the PAT may be too strict for some box materials. The air space in the box does somewhat mitigate the harmful effects from the high-lignins boxboards. The test results, however, do not answer questions about how these effects might vary as box volumes change or how the boxboard might affect photographs when the box is filled. There is also the possibility that the inclusion of an alkaline reserve in the boxboards may reduce these harmful effects enough to pass the box test. So, until further research can be performed, storage boxes, slipcases, and album binders should ideally be made of the same quality paper products as enclosures intended for direct contact with the image. 6 CONCLUSIONSThe following conclusions about paper and paperboard enclosures were drawn:
7 RECOMMENDATIONS FOR FUTURE RESEARCHIt was the original intent of the project to examine lignins-containing enclosures from the perspective of selecting new products for use in collections. While anecdotal evidence had already resulted in most institutions switching to lignins-free enclosures, the basis for this change had yet to be established experimentally. The reality exists, however, that there are already many lignins-containing enclosures in collections. The photographic materials were either housed before the collection managers understood the need for lignins-free enclosures or housed prior to being received into the collection. The question arises, then, regarding the priority of improving storage conditions over rehousing materials already in poor-quality enclosures. It is logical that reducing temperature and humidity of the storage environment will slow the harmful reactions between lignins-containing enclosures and photographs. What is not known is the amount of improvement that any change in storage conditions will make. However, reducing ambient temperature and moisture has the added bonus of reducing the decay rates of the objects themselves. It follows that developing good storage conditions may be the priority over rehousing. Future research examining the effect of temperature and humidity on ligninsphotograph reaction rates could give collection managers a better sense of time with regard to making rehousing decisions. Future research might also examine further variations in box materials including the potential reactivity of semipulp, buffered semipulp, or buffered groundwood boards on photographs or the potential advantages of using various liner materials inside the box to absorb or block the harmful volatiles from the lignins-containing core. ACKNOWLEDGEMENTSThe authors gratefully acknowledge Creative Memories for their support in the preparation of this manuscript and the American Society for Testing and Materials Institute for Standards Research for the several papers used in this study. REFERENCESAdelstein, P. Z., J. M.Reilly, D. W.Nishimura, C. J.Erbland, and J. L.Bigourdan. 1995. Stability of cellulose ester base photographic film. Part 5, Recent findings.Society of Motion Picture and Television Engineers Journal104:439–47. ASTM. 1996. Determination of alkalinity of paper as calcium carbonate (alkaline reserve test of paper), D 4988–96. Philadelphia: American Society for Testing and Materials. B�gin, P., S.Desch�telets, D.Grattan, N.Gurnagul, J.Iraci, E.Kaminska, D.Woods, and X.Zou. 1998. The impact of lignin on paper permanence: A comprehensive study of the aging behavior of handsheets and commercial paper samples.Restaurator19:135–54. Biermann, C. J.1996. Handbook of pulping and papermaking. San Diego: Academic Press. Carroll, J. F., and J. M.Calhoun. 1955. Effect of nitrogen oxide gases on processed safety film.Society of Motion Picture and Television Engineers Journal64:501–7. Collings, T. J., and F. J.Young. 1976. Improvements in some tests and techniques in photograph conservation.Studies in Conservation21:79–84. ISO. 1999. Photography: Processed photographic materials, Photographic activity test for enclosure materials, 14523:1999. Geneva: International Organization for Standardization. Oddy, W. A.1973. An unsuspected danger in display.Museums Journal73:27–28. Oddy, W. A.1975. The corrosion of metals on display. In Conservation in archeology and the applied arts,ed. D.Leigh et al. London: International Institute for Conservation of Historic and Artistic Works. 235–37. Schmidt, J. A., C. S.Rye, and N.Gurnagul. 1995. Lignin inhibits auto-oxidative degradation of cellulose.Polymer Degradation and Stability49:291–97. TAPPI. 1988. Solvent extractives of wood and pulp, T204 om-88. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1993. Fiber analysis of paper and paperboard, T401 om-93. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1996. Hydrogen ion concentration (pH) of paper extracts (cold extraction method), T509 om-96. Atlanta: Technical Association of the Pulp and Paper Industry. SOURCES OF MATERIALSChipboardCampus Connections Rochester Institute of Technology 1 Lomb Memorial Dr. Rochester, N. Y. 14623 Conservation board and PVA glueLight Impressions P.O. Box 787 Brea, Calif. 92822-0787 AUTHOR INFORMATIONDANIEL M. BURGE received his bachelor of science in photographic and imaging technology from the Rochester Institute of Technology in 1991 and is currently a research scientist at the Image Permanence Institute. Address: Image Permanence Institute, Rochester Institute of Technology, Rochester, N.Y. 14623. JAMES M. REILLY graduated with a B. A. from Franklin and Marshall College in 1968 and an M. A. from the State University of New York at Buffalo in 1972. He has continued his education in science at the Rochester Institute of Technology. In 1984 he was appointed director of the Photo Preservation Laboratory at the Rochester Institute of Technology, and the following year he organized and was made director of the Image Permanence Institute at the same institution. He is also a full professor in the College of Imaging Arts and Science. Address as for Burge. DOUGLAS W. NISHIMURA received his bachelor of science in chemistry from McMaster University in Canada. He worked for the Public Archives of Canada (National Archives of Canada) from 1983 to 1986 under the direction of Dr. Klaus B. Hendriks. From 1986 to the present, he has been a senior research scientist at the Image Permanence Institute. Address as for Burge.
 Section Index Section Index |