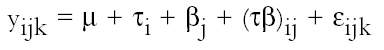ANTIFUNGAL PROTECTION AND SIZING OF PAPER WITH CHITOSAN SALTS AND CELLULOSE ETHERS. PART 2, ANTIFUNGAL EFFECTSMARIA DEL PILAR PONCE-JIM�NEZ, FERNANDO A. L�PEZ-DELLAMARY TORAL, & HUMBERTO GUTIERREZ-PULIDO
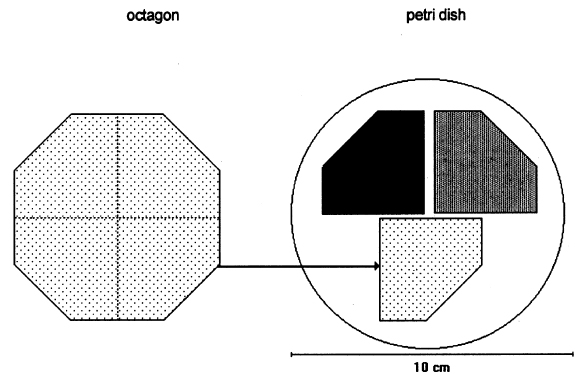
ABSTRACT—In search of alternatives for the professional conservator to use in the struggle against fungi that deteriorate paper documents, we decided to research chitosan, given its antimolding and strengthening properties. This, the second of two articles on the results, reports on the antifungal effects. We used samples of Whatman no. 1 filter paper sized with three chitosan salts—chitosan acetate (AQ), chitosan butyrate (BQ), and chitosan propionate (PQ)—and three cellulose ethers— carboxymethylcellulose (CMC), methylcellulose (MC), and hydroxypropylmethylcellulose (HPMC). They were sterilized with ethylene oxide (EtO) and inoculated with Chaetomium globosum, Aspergillus niger, Aspergillus terreus, Alternaria alternata, Penicillium sp., and Chaetomium britannicum spores. After the biological tests, chitosan salts showed considerably more antifungal resistance and improved resistance in paper compared with cellulose ethers. TITRE—Protection anti-fongique et encollage du papier au moyen du chitosane et des �thers cellulosiques. 2e partie, les propri�t�s antifongiques. R�SUM�—�tant donn� ses qualit�s antifongiques et renforcissantes, une �tude a �t� entreprise afin de d�terminer si le chitosane pourrait �tre utilis� lors du traitement des documents en papier atteints de moisissure. Ceci est le deuxi�me article sur ce sujet et il discute des propri�t�s antifongiques. Des �chantillons de papier filtre Whatman no. 1 ont �t� enduits de trois sels de chitosane, soit l'ac�tate de chitosane (AQ), le butyrate de chitosane (BQ) et le propionate de chitosane (PQ), ainsi que de trois �thers cellulosiques, soit la carboxym�thylcellulose sodique (CMC), la m�thylcellulose (MC) et l'hydroxypropylm�thyl cellulose (HPMC). Les �chantillons ont �t� st�rilis�s avec de l'oxyde d'�thyl�ne et inocul�s avec les spores des moisissures suivantes: Chaetomium globosum, Aspergillus niger, Aspergillus terreus, Alternaria alternata, Penicillium sp. et Chaetomium britannicum. Apr�s ces tests biologiques, les �chantillons enduits avec les sels de chitosane d�montr�rent des propri�t�s antifongiques consid�rablement accrues, ainsi qu'une meilleure r�sistance m�canique comparativement aux �chantillons enduits d'�thers de cellulose. TITULO—Protecci�n contra hongos y apresto de papel con sales de quitos�n y �teres de celulosa. Parte 2, efectos contra los hongos. RESUMEN—En busca de alternativas de uso para el conservador profesional en la lucha contra los hongos que deterioran los documentos de papel, decidimos investigar el quitos�n, considerando que tiene propiedades fungicidas y que refuerzan el papel. En este segundo art�culo, de dos que escribimos sobre el tema, se analizan los resultados de los efectos contra los hongos. Se recubrieron muestras de papel de filtro Whatman no. 1 con sales de quitos�n —acetato de quitos�n (AQ), butirato de quitos�n (BQ), y propionato de quitos�n (PQ)—y tres �teres de celulosa—carboximetil celulosa de sodio (CMC), metil celulosa (MC), e hidroxipropil metil celulosa (HPMC). �stos fueron esterilizados con �xido de etileno e inoculados con esporas de Chaetomium globosum, Aspergillus niger, Aspergillus terreus, Alternaria alternata, Penicillium sp., y Chaetomium britannicum. Despu�s de realizadas las pruebas biol�gicas, en comparaci�n con otros �teres de celulosa, las sales de quitos�n mostraron una marcada resistencia contra los hongos y mejoramiento de la resistencia en el papel. 1 INTRODUCTIONPart 1 of this article showed the physical and mechanical changes in samples of Whatman no. 1 filter paper consolidated with cellulose ethers and chitosan salts (Ponce-Jim�nez et al. 2002). In this article, we compare the antifungal protection of these consolidants against six selected fungal strains. When a paper object has been attacked by fungi, the deterioration is shown by color spots, weakening of the fibers due to depolymerization of cellulose, and shifts in pH to acidic. Development of filaments or hyphae and proliferation of spores may also occur. This situation is deleterious because even after fumigation, latent spores can remain in the paper and The paper conservator must use the most effective substances for the protection of documents and works of art damaged by fungi. Conserving the paper object while minimizing collateral risks to people and to other objects is the most critical goal. It is urgent to exterminate fungi immediately and achieve the best possible protection (Dersarkissian and Goodberry 1980; Santucci 1983). The cellulose ethers—for example, carboxymethyl-cellulose and methylcellulose—are the substances used most frequently in restoration and conservation of paper, mainly as sizing and reinforcing agents, but cellulose ethers are susceptible to enzymatic degradation. This effect is manifested in the reduction of the molecular mass. Only cellulose ethers with a high substitution degree and uniform substitution pattern along the chain can be more resistant against microbial degradation (Strnadov� and Dur�vic 1994). 1.1 ANTIFUNGAL PROPERTIES OF CHITOSANLeuba and Stossel (1986) and Hardwiger et al. (1986) demonstrated the antifungal effect of chitosan on some plant root diseases. While chitin and chitosan both have antifungal properties, chitosan is more effective. Chitin suppresses fungi indirectly via antagonistic soil micro-organisms, while chitosan has both direct and indirect effects (Leuba and Stossel 1986). It can activate specific genes in plants and, at similar concentrations, can completely inhibit RNA synthesis in some fungal organisms (Hardwiger et al. 1986). Chitosan can inhibit both Epidermophyton floccosum, an athlete's foot–inciting fungus, and Ceratocystis ulmi, the causal agent of Dutch elm disease, which attacks certain kinds of trees (Hardwiger et al. 1986). Chitosan treatments of wheat seeds prior to planting can afford the wheat plant protection from some of the detrimental effects of root pathogens that cause lodging (fallen wheat) and yield loss (Hardwiger et al. 1986). Hardwiger et al. (1986) attribute the biological activity of chitosan to interaction among positively charged amino groups of chitosan and negatively charged phosphate groups of nucleic acids. The chitosan polymer must be seven or more sugar units in length to optimally induce plant genes and inhibit fungal growth. This length requirement suggests that a series of positive charges match up with phosphate negative charges in the grooves of the DNA helix in the B form, possibly converting it to the Z form (Hardwiger et al. 1986). Chitosan agglutinates a variety of bacteria and yeasts as well as cells of mammalian origin (Leuba and Stossel 1986). Chitosan is also a component in the wall of some fungi, species that are less sensitive to its antifungal effects (Leuba and Stossel 1986). These fungi presumably alleviate the potential destruction of chitosan through exclusion, rapid degradation, and so on. (Some species of fungi [e.g., Fusarium] have some potential to regulate chitosan levels, preventing fungal or germination growth for defined periods [Hardwiger et al. 1986].) Chitosan also accumulates in dormant chlamydospores, but the level is much lower in mycelia following germination of these spores. Thus, chitosan may be utilized by the fungus as a natural regulator molecule to manipulate the dormant state (Hardwiger et al. 1986). In this article, we evaluate the effect of three chitosan salts as antifungal consolidants for potential applications in conservation treatments of documents previously attacked by fungi and as a preventive against deterioration. In tests, six celluloytic fungal strains were employed with previous evaluation of their effects on Whatman no. 1 filter paper. 2 METHODOLOGYThe methodology we used for the antifungal tests was inspired by TAPPI standard T487 om-93 (TAPPI 1993a), which is a method for determining antifungal resistance of paper and paperboard, mainly when they have been chemically treated against fungi. The standard describes two methods: the “spore-mycelia” TAPPI standard T487 om-93 (TAPPI 1993a) recommends using three strains of fungi of the species: Chaetomium globosum, Aspergillus niger, and Aspergillus terreus. Unfortunately, the specific strains are very difficult to obtain, but the same species of test organisms were employed. 2.1 SAMPLES OF PAPERWhatman no. 1 filter paper was used because it is a pure cellulose paper, free of additives, and a point of reference in respect to other experimental works. The choice of paper samples to size with polymers was done in accordance with TAPPI standard T400 om-90 (TAPPI 1990). The shape of the filter paper used for the samples was different from that indicated in the standard. For example, 420 mm2 octagons were used in place of 50 mm2 squares (see appendix for other modifications). After the polymer sizing dried, each octagon was separated into four sections, forming pentagons of 105 mm2. Three samples were placed in each Petri dish (fig. 1). Each sample was numbered with a graphite pencil according to the numbers in table 1 that indicate the kind of sizing and the fungal strain assigned. Each sample was also marked with lines in the cross direction of the paper, with 1.5 cm separation between lines. For the antifungal test, one-fourth of an octagon was used for each sample; for the zero-span tensile strength, strips 1.5 cm wide were used. Testing was done along the cross direction of the paper.
After sizing, the samples were disinfected with ethylene oxide (EtO) as described below and were placed in Petri dishes as shown in figure 1. Each section of paper had a different consolidant. The samples were injected with a 2% solution of each of the polymers indicated in section 2.2. After the samples were sized with a roll hand-coater no. 8 (for printing ink tests), they were left to dry over a nylon screen and then neutralized using ammonia vapors in a closed chamber for 20 minutes. Sterilization of the paper samples was done in a hospital clinic using ethylene oxide. This was the method that least affected properties of the paper (autoclave and ozone were also evaluated for sterilization, but autoclave decreases the resistance and brightness of paper, and ozone is not effective). 2.2 CONSOLIDANT SIZESThe chitosan was prepared from deproteinized and deacidified shrimp shells by treatment with alkali in two steps to diminish its content of N-acetyl groups (percentage of free primary amines). The acetylation degree of the sample was 17.3%; the viscosity (a capillary viscometer) of a 1% (w/v) in 1% (v/v) acetic acid solution was 51 cps; and its content in ashes was 0.23% (L�pez-Dellamary 1996). Three different 2% (w/v) solutions of chitosan were used, employing in each case glacial acetic acid, butyric acid, and propionic acid to dissolve the chitosan. Thus we prepared three salts: chitosan acetate (AQ), chitosan butyrate (BQ), and chitosan propionate (PQ). The preparation consisted of adding 2 g of shredded chitosan to 100 ml of deionized water, then stirring the mixture while adding drop by drop 1 ml of the corresponding acid to completely dissolve the chitosan. Preparation of 2% solutions of chitosan salts and cellulose ethers is described in part 1 of this article (Ponce-Jim�nez et al. 2002).
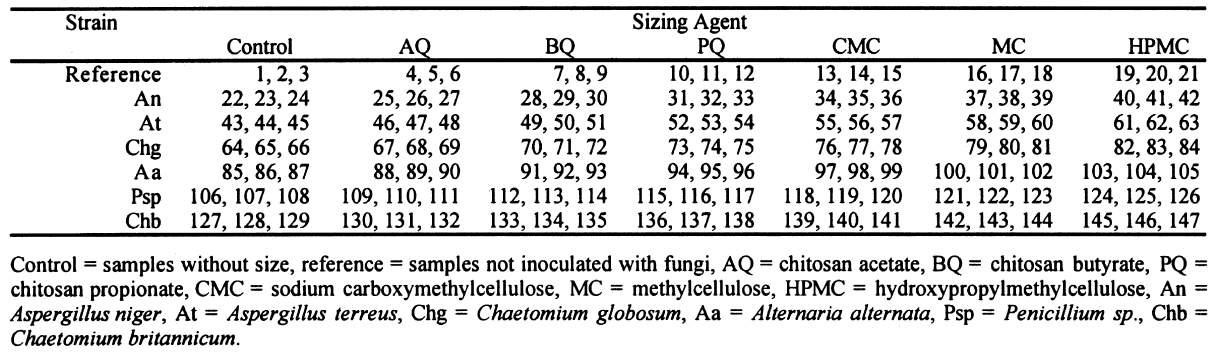
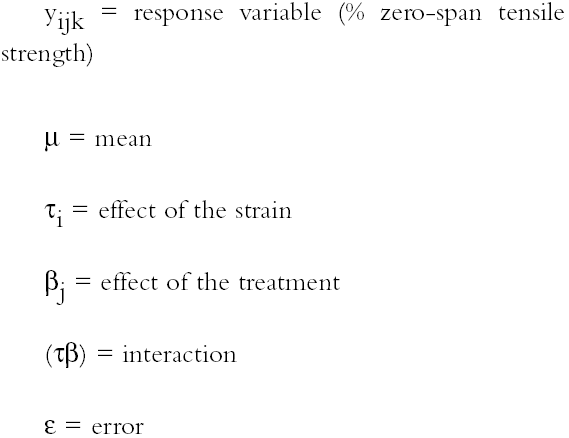
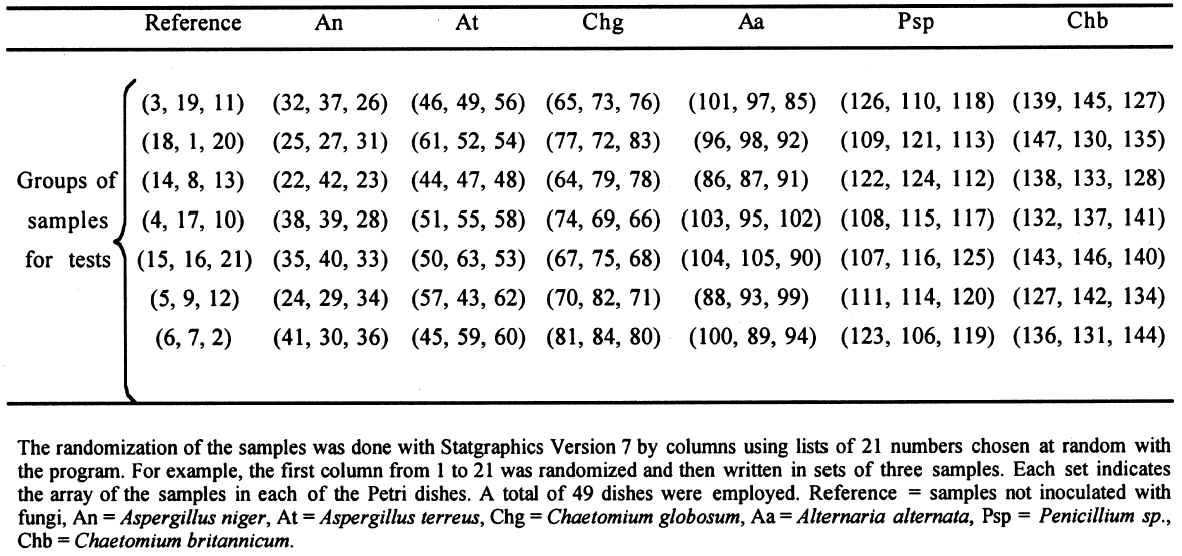
2.3 FUNGAL STRAINSIn the preliminary stages of this work, we did cellulolytic activity tests on 16 strains that were isolated from 17th–20th-century documents from the Archive Historical of Notaries, Mexico City, that showed deterioration by fungi. Two different techniques were used to make our determination: loss of mass and degree of polymerization (see appendix). Both tests were done on pulp samples of Whatman no. 1 filter paper with the 16 isolated strains. The results of both tests nearly coincide in the cellulolytic activity of each strain. Three of the most cellulolytic strains were selected for the biotests and identified in the Mycology Laboratory of Polytechnic National Institute, Mexico City, as Alternaria alternata, Penicillium sp., and Chaetomium britannicum. Methods of determining percentage of mass loss and degree of polymerization are described in the appendix. In the tests for antifungal protection, the following strains of fungi were utilized to induce deterioration on the paper: Chaetomium globosum (Chg), Aspergillus niger (An), Aspergillus terreus (At), Alternaria alternata (Aa), Penicillium sp. (Psp), and Chaetomium britannicum (Chb). Reference samples for the tests were not inoculated with fungi. The control samples in the group of treatments or coatings were without any type of size consolidant. All strains were grown on potato-dextrose-agar medium at 28�C and 100% RH in Petri dishes and culture tubes. The strains were grown in test tubes for 14 days, after which suspensions of the spores and mycelium were prepared and inoculated on the paper samples in accordance with TAPPI standard T487 om-93 (TAPPI 1993a), with some slight variations (see appendix). To determine the antifungal resistance, the EtO-sterilized polymer-coated paper samples were placed on agar mineral salts, and 15 ml of culture medium was placed in each Petri dish. Later, the samples of paper were put on the solid medium in a sterile field, and they were kept in hermetic boxes at 28�C. After 24 hours, 1 ml of each spore-mycelia suspension was inoculated with sterile disposable syringes in the sterile field inside a laminar flow chamber (hood). A new syringe was used to inoculate each strain. During incubation, the samples were placed in hermetically closed plastic boxes. The Petri dishes were arrayed in reverse position on a raised screen to form a space that allowed a volume of water to be retained in the bottom of the box, maintaining an RH near 100%, with a temperature of 28�C, and in darkness. The samples were kept completely moist for the total incubation period of 21 days. 2.4 TREATMENT OF THE SAMPLES WITH 70% ETHYL ALCOHOL (ASEPTIZATION)To be able to handle the samples aseptically after 2.5 ZERO-SPAN TENSILE STRENGTH AS A TEST OF DETERIORATIONThe zero-span tests were done with a modified pendulum-type tensile strength machine, and strips 1.5 cm wide were utilized in accord with TAPPI T231 cm-85 (TAPPI 1985). Zero-span tensile strength is different from folding endurance in that it is related more to the intrinsic resistance of the fibers than to the cohesive force between them. So measuring the force necessary to break the paper shows the resistance of the fiber, not the resistance of the fiber-to-fiber bonding. The protection given by the consolidants was evaluated by zero-span tensile strength because the intrinsic resistance of the fibers is in intimate relation with its state of conservation. Submitting the paper to enzymatic or aging deterioration will affect the zero-span tensile strength. 2.6 FACTORIAL EXPERIMENTAL DESIGN 7 X 7To determine the effect caused by each one of the strains and their combined effect with each one of the coatings, we did a factorial experimental design of 7 � 7 factors, obtaining 49 different combinations. In table 1, the column heads list the kinds of coating, and the row headings list the types of fungi strain. In table 2 the order of assignment of each Petri dish is indicated. This order was obtained by randomizing the total number of samples (three samples were placed in each dish). The mathematical model for this experimental design is:
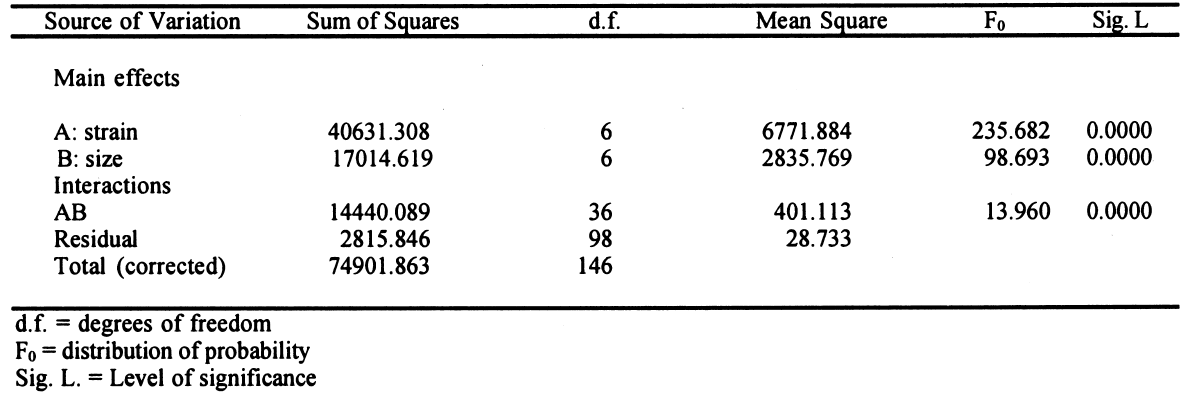
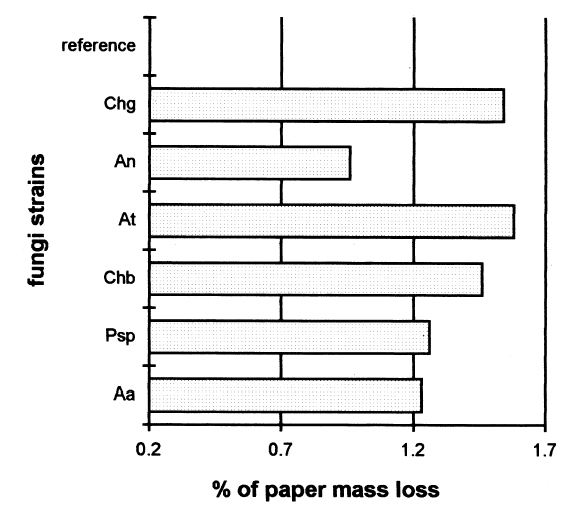
Each test was repeated three times. The total number of samples was calculated as follows: 7 � 7 = 49 (3 repetitions) = 147 samples. This design is applied to each response variable, in this case for the percentage of zero-span tensile strength. At the end of the experimental treatment, the readings of zero-span tensile strength were put into a computer and processed using Statgraphics version 7, filling three columns with data: (1) type of strain, (2) consolidant size, and (3) zero-span tensile strength. Analysis of variance (ANOVA, for more than one variable) was done for strain, size, and their interactions (table 3). Figures 2 and 3 compare the means. The interaction effect of size and strain on zero-span tensile strength is shown in figure 4. 3 RESULTS3.1 RESULTS OF FUNGAL ATTACKIn figure 5, the horizontal bars correspond to the percentage of mass loss after the fungal attack. The length of the bars is equal to the cellulolytic activity of each strain. Included in the graph is a reference that had 0% mass loss.
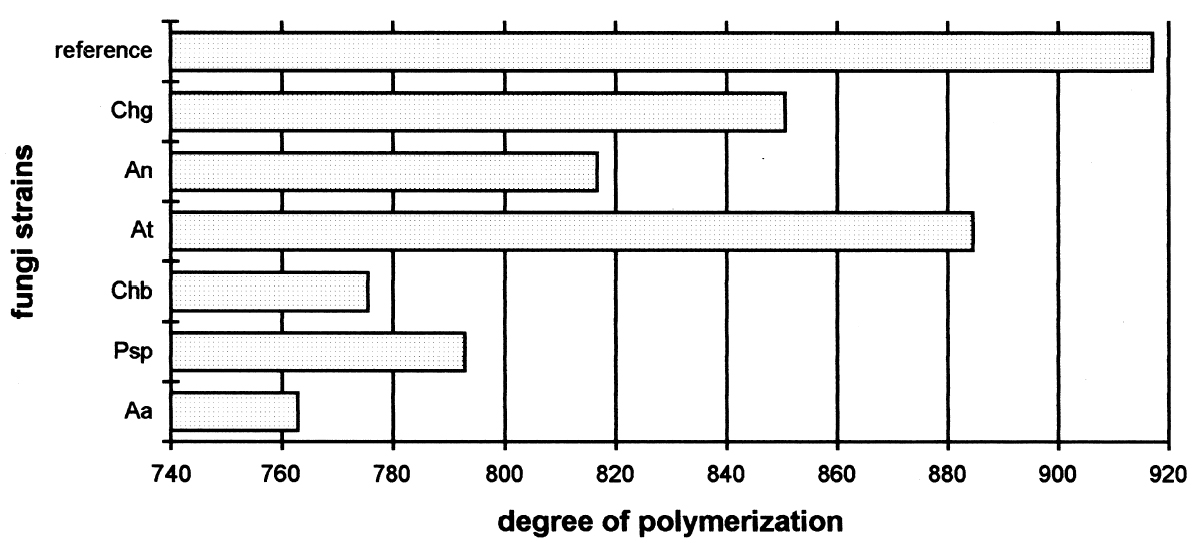
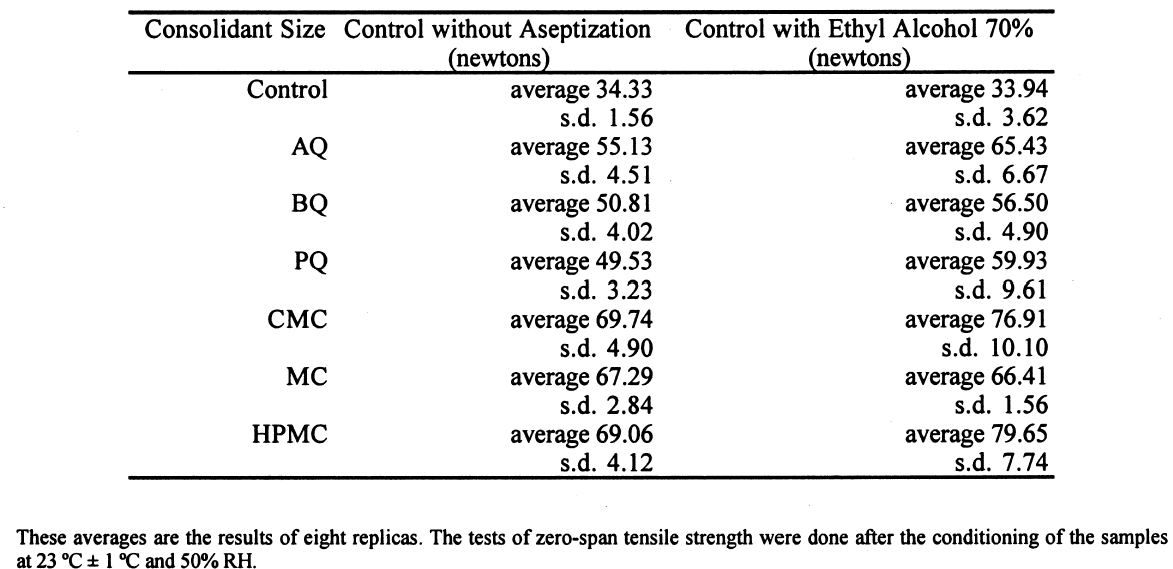
Figure 6 shows the degree of polymerization after 21 days of fungal attack. The longer bars are those with less deterioration. The length of the horizontal bars is inversely proportional to the cellulolytic activity of each strain. The longest bar represents the average degree of polymerization in reference samples without fungi. 3.2 RESULTS OF THE TREATMENT WITH 70% ETHYL ALCOHOLThe influence of alcohol on paper strength was evident, increasing the standard deviation of all the samples. The sized samples, except that with the MC size, have a general tendency to increase zero-span tensile strength (table 4). The alcohol treatment slightly altered the results, but it was the only feasible option for stopping the fungal activity and handling the samples after the biotests. Treatment with formaldehyde could greatly alter the properties of the consolidant sizes. In this stage of the experiment, an ETO sterilization chamber was not available.
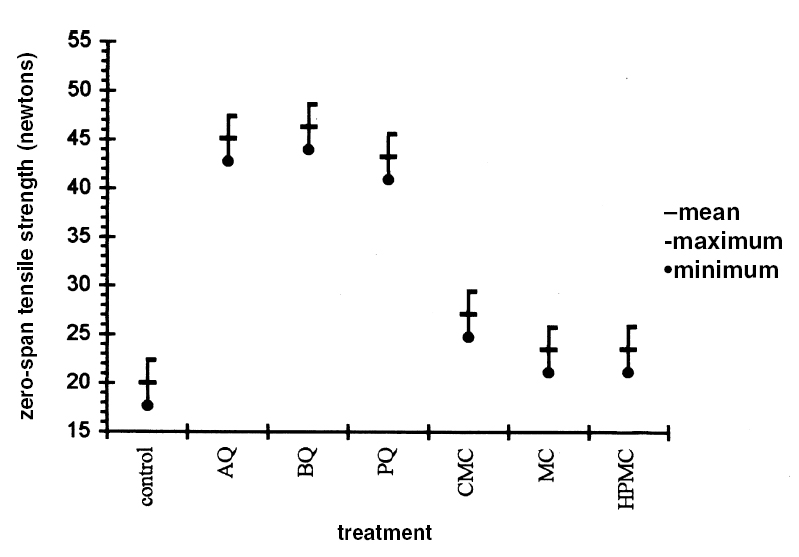
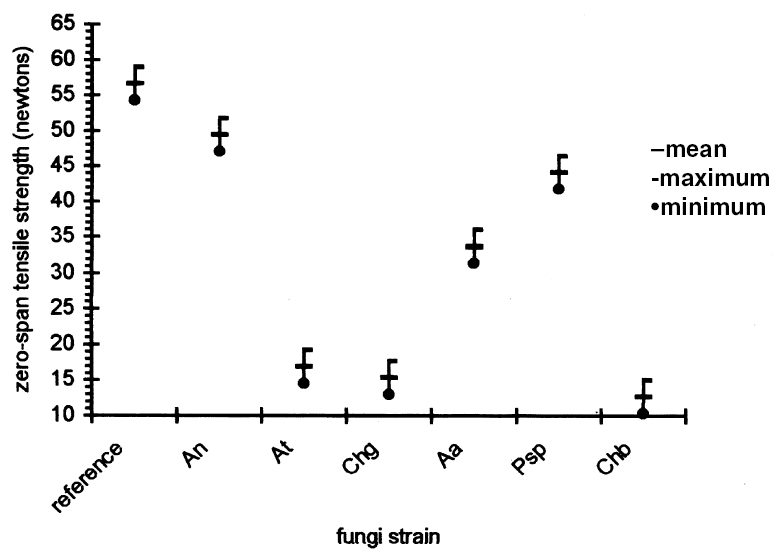
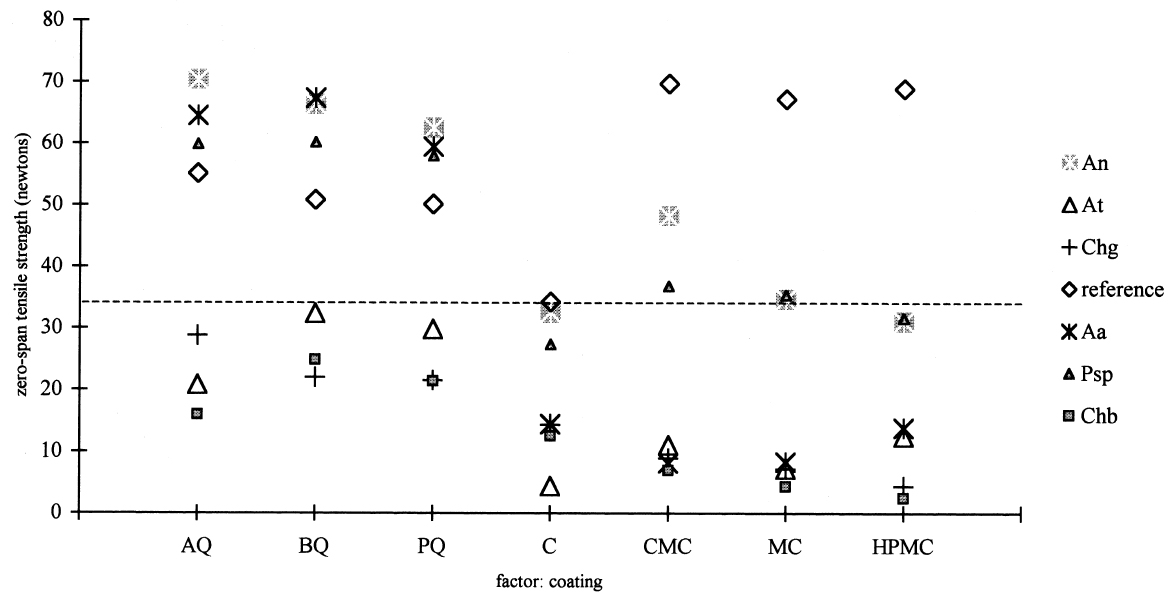
3.3 RESULTS OF THE EXPERIMENTAL DESIGNIn this experiment we evaluated two factors, strain and sizing, and each one at six levels. We used two types of sizes, the chitosan salts (Q) and the cellulose ether (E). The level of each factor—strain and coating— was considered. With seven levels for each factor, including the controls and references, analysis of variance was an appropriate standard test. Zero-span tensile strength was used to evaluate deterioration, because we observed that all the strains grew on the paper. The control samples showed the maximum development of hyphae, while the least concentration of hyphae was in samples treated with Q. The samples with E show a high density of hyphae. It was impractical to determine the percentage of invasion. The ANOVA results presented in table 3 indicate significant differences between the levels of strain and size, and there are interactions between both factors. Figures 2 and 3 show the average effects of each level for both factors (type of size and type of strain). Figure 2 shows clearly that the samples treated with AQ, BQ, and PQ had less deterioration than samples
The zero-span tensile strength of all the samples was compared for all the types of coatings, including the control, after they had been submitted to the attack of the fungal strains. We observed that, in general, Aspergillus terreus, Chaetomium globosum, and Chaetomium britannicum caused the most deterioration of the samples, while Aspergillus niger and Penicillium sp. had less effect on the zero-span tensile strength. Strain Alternaria alternata caused an intermediate loss in the zero-span tensile strength of the paper (figs. 3, 4). These averages are the results of eight replicas. The tests of zero-span tensile strength were done after the conditioning of the samples at 23�C � 1�C and 50% RH.
4 DISCUSSIONThe combination of many kinds of variables was necessary because of the natural demands of solving the problem of conserving documents from attack by fungi. Also to be taken into consideration is the added complexity of considering the inks, dyes, and pigments that form part of the documents. Sterilization of the samples after applying the polymers was necessary to ensure that only test organisms and no other contaminants interacted with the paper. To evaluate the protective effect of the sizes after the fungal attack, it was necessary to submit the samples to an aseptic step, though one not equal to fumigation. This procedure helped us to handle the attacked material with some confidence and served as a precaution against dispersal of spores that could enter researchers' respiratory tract. These aseptic steps influenced the results of zero-span tensile strength, having a tendency to reinforce this property of the paper. This had little effect on the final results. Results of zero-span tensile strength were analyzed with Statgraphics Version 7. The ANOVA results summarized in table 3 show that the factors of strain and coating have significant differences in effect on zero-span tensile strength in the different variations. There is also an interaction between the two factors because the protective effect of each treatment depends on the strain that is being tested. Figure 4, in which the “x” axis represents the kind of coating and each group of points shows a different kind of strain, demonstrates clearly that chitosan salt coatings offer more protection than cellulose ether coatings do. But important deterioration occurred in both, probably as a consequence of uneven coating. The graph also shows that the reference samples sized with cellulose ethers have higher zero-span tensile strength than reference samples sized with chitosan salts. We do not know the protection mechanism of chitosan on paper. To help clarify this mechanism, future testing to evaluate the toxicity of chitosan on specific strains of fungi is needed. It is possible that protection is created by the formation of a barrier shielding the fibers against degradation, and we cannot discard the possibility of a true inhibition. Chitosan salts AQ, BQ, and PQ reacted so similarly that it is not possible to distinguish the margin of difference. Cellulose ethers also reacted very similarly, although HPMC and MC have more similarity in their properties and reaction than they do with CMC, even though the three products function almost equally. However, the cellulose ether coatings exhibited little protection. Apparently CMC was more resistant to fungal attack than MC and HPMC were (see figs. 2, 4). Dobroussina et al. (1996) mention that in papers coated with some types of cellulose ethers, micro-organism damage can occur. Although the development time is much slower than in the uncoated paper, it is thought that the attack occurs only when there are microscopic holes on the coated surface, which generally happens when one polymer is applied in solution or melted. Maybe if there were more viscosity (see Ponce-Jim�nez et al. 2002), cellulose ethers would be more likely than the chitosan salts to form an irregular film with large pores. However, it is enormously important to consider the differences between fungi strains. Obviously none of the materials examined have the capacity to inhibit all kinds of fungi. And there is a common association between fungi and bacteria in the deterioration of materials like paper with a low nutrient content (Mandels and Reese 1960). Also, the additives often increase the nutritive value of the material, reinforcing the fungal attack. Even though short-term protective effects can be gained, there is no guarantee of long-term effectiveness for chitosan, for which it will be necessary to design new investigations. It was observed that the strains with higher cellulolytic activity were, from high to low, Chaetomium britannicum, Chaetomium globosum, and Aspergillus terreus. The analysis of figure 4 led to the following conclusions: the Alternaria alternata strain shows notable variables according to the type of chitosan salt or cellulose ether applied, being more aggressive to the control, HPMC, MC, and CMC than to chitosan salts. The strain that caused the most damage was Chaetomium globosum. 5 CONCLUSIONSChitosan is of interest for the field of conservation, probably not only for paper but also for other materials such as skin, leather, parchments, wood, textiles, and photographs. Some investigations have been done with wood and textiles (Sadov and Markova 1954; Furukawa and Yamamoto 1990; Kumagai et al. 1990; Lee et al. 1993). Chitosan products protect paper from deterioration more than cellulose ethers do. The probable mechanism is a mechanical barrier that could impede penetration of hyphae. At the same time, a real inhibition of the growth of fungi can occur, but the degree of protection depends greatly on the kind of fungi attacking. It is important to evaluate chitosan's toxicity to specific strains of fungi so the protection mechanism of chitosan on paper can be clarified. The zero-span tensile strength test is a sensible means to grade the deterioration of paper, as much as it is a good parameter to test the effectiveness of the antifungal treatment afterward. Sterilization before inoculation of the paper with fungi was necessary to avoid contamination and for specific evaluation of each strain. The prospect of employing products of chitosan in considering the techniques of document conservation will be interesting, since they simultaneously protect against attacks by micro-organisms and reinforce the paper's physical strength. Obviously we do not yet have a perfect antimold consolidant for paper, but our results show that we are closer to finding one, because chitosan can be modified and can produce many different derivatives to improve its properties for specific applications. We hope this work will spark interest in the possibilities of using chitosan for the conservation of artwork and historical objects. APPENDIXVariations to TAPPI Standard T487 om-93The standard describes two methods, the “spore-mycelia” and the “cloud of spores.” Only the spore-mycelia method was used. Modifications consist of: 1. The form of the filter paper used for the samples was different from that indicated in the standard. In place of squares of 50 mm2, octagons of 420 mm2 were used. After the polymer coatings dried, each octagon was separated into four sections, each with an area of 105 mm2. 2. Fungi of a species recommended by TAPPI standard T487 om-93 were employed for test organisms, but not the exact strains because the exact ones were not obtainable. 3. Incubation period for the inoculated samples was 21 days instead of 14 days, at 28�C and 100% RH. 4. In place of observing presence or absence of filaments or colonies of fungi, evaluation of results was in values of zero-span tensile strength (newtons). Then, after drying, the samples were cut in strips 1.5 cm wide (see TAPPI 1985). Percentage of Weight LossFive g of Whatman no. 1 filter paper was shredded in aseptic conditions. The shredded material was placed in a blender with the blades protected by duct tape to prevent fiber degradation. Enough deionized water was added to obtain a fiber slurry. This substance was then transferred to a glass filter, and water was removed by suction. The pulp was lifted out with a spatula, homogenized, and bagged and sealed in polyethylene. Moisture content was measured in a thermal balance. For the next step, 5 g (oven-dry basis) samples were weighed and placed in 50 ml wide-mouthed glass jars, capped, and sterilized in an autoclave at 10.42 kilopascals for 15 minutes. Strain CultureEach strain was inoculated in a culture test tube with potato-dextrose-agar medium and was incubated 21 days until sporulation. To inoculate the paper samples, each strain was prepared as follows. First, glass jars containing 100 ml of distilled water and 30 glass beads were autoclaved. Then, to each test tube, 5 ml of sterile water was added, and the mycelium was scraped with a wire loop, followed by vigorous shaking to produce a fragmented mycelium and spore slurry. The contents were then poured into the glass jars of sterile distilled This process was repeated for each strain. Later, 1 ml of the slurry was taken up with a sterile plastic syringe and applied to the paper pulp previously stored in the glass jars. The jars were immediately capped and incubated at 28�C for 28 days in a sealed plastic box containing a water layer in the bottom as a reservoir to keep relative humidity at 100%, monitored by a hygrometer. After this time period, 70% ethyl alcohol was added to kill the fungi, then the sample was dispersed using a mixer with distilled water. It was then filtered through a glass filter with suction, washed with 70% ethyl alcohol, placed in small aluminum dishes and air-dried, and then oven-dried at 105�C until reaching constant weight. Results are reported in percentage of weight loss according to the following formula:
Degree of PolymerizationThe paper samples and inoculation strains were prepared in the same manner as the tests for percentage of weight loss. Evaluating degree of polymerization was in accordance with ASTM standard method D4 243-99 (1999). To do the viscosity determination, each sample was dissolved in a cupriethylenediamine solution, and the fluid velocity was measured in a viscometer. Ten replicates of each sample were made, and the averages were calculated. Results of the selected strains are shown in figure 6. ACKNOWLEDGEMENTSWe wish to thank the following persons for their support and for making this work possible: Karl Augustin Grellman Institute of Wood, Cellulose and Paper, University of Guadalajara, Guadalajara, Jalisco: Jos� Turrado Saucedo, chemical engineer and head of the technology laboratory; Jos� de Jes�s Rivera Prado, chemical engineer, the bleaching laboratory; Salvador P�rez Ramos, chemical engineer, the physical-mechanical tests laboratory; Francisco Vel�zquez Cervantes, chemical pharmacology biologist, and Leticia Maya, of the chemistry laboratory; Professor Bruno Becerra Aguilar of the technology area; Hilda Palacios Ju�rez, chemical engineer, the microscopy laboratory; and Luz Elena Arce Castillo, chemical engineer, the library and publications area. Biologist Rosario V�squez of the Mycology Laboratory of Polytechnic National Institute in Mexico City. University Center of Biological and Agronomical Sciences, University of Guadalajara: Professor Rosa Mar�a Dom�nguez Arias. Mexican Institute of Social Security Clinic 46 in Guadalajara: Maria Luisa Preciado Quiroz and Mar�a de Jes�s Najar. Mercedes G�mez Urquiza, director of the Manuel del Castillo Negrete National School of Conservation, Restoration and Museology; Luis Daniel Mario Goeritz Rodriguez, engineer and director of the National Institute of Anthropology and History Veracruz Center. We also thank Daniel McGonagle for assistance in translating part of the text. REFERENCESASTM. 1999. Standard method for measurement of average viscometric degree of polymerization of new and aged electrical papers and boards. D4243-99. Philadelphia: American Society for Testing and Materials. Dersarkissian, M., and M.Goodberry. 1980. Experiments with non-toxic antifungal agents.Studies in Conservation25:28–36. Dobroussina, S. A. D., T. D.Velikova, and O. V.Rybalchenko. 1996. A study of the biostability of parylene-coated paper.Restaurator17:75–85. Furukawa, Y., and S.Yamamoto. 1990. Improvement of wood quality with chitin and chitosan. Part 2, Assessment of the fungicidal effect of chitosan-treated wood on wood decaying fungi and microorganisms in the soil.Research Bulletin of the Tottori University Forests19:49–65.
Hardwiger, L. A., D. F.Kendra, B. W.Fristensky, and W.Wagoner. 1986. Chitosan both activates genes in Kumagai, H., Y.Furukawa, T.Sakuno, and J.Kishimoto. 1990. Anti-mold activity of chitosan-treated wood: Evaluation of anti-mold activity of chitosan and chitosan/copper sulfate jointly treated wood.Research Bulletin of the Tottori University Forests19:59–65. Lee, J. S., Y.Furukawa, and T.Sakuno. 1993. Preservative effectiveness against Tyromyces palustris in wood after pretreatment with chitosan and impregnation with chromate copper arsenate. Mokuzai Gakkaishi. Journal of the Japan Wood Research Society39(1):103–8. Leuba, J. L., and P.Stossel. 1986. Chitosan and other polyamines: Anti-fungal activity and interaction with biological membranes. In Chitin in nature and technology, ed. R. A. A.Muzzarelli, C.Jeuniaux, and G. W.Gooday. New York: Plenum Press. 215–22. L�pez-Dellamary, F. A.1996. Personal communication. Chemistry Laboratory, Karl Augustin Grellman Institute of Wood, Cellulose, and Paper, University of Guadalajara, Guadalajara, Mexico. Mandels, M., and E. T.Reese. 1960. Induction of cellulase in fungi by cellobiose.Journal of Bacteriology79:816–26. Ponce-Jim�nez, Maria del Pilar, Fernando Lop�z-Dellamary Toral, and Ezequiel Delgado-Fornu�. 2002. Antifungal protection and sizing of paper with chitosan salts and cellulose ethers. Part 1, Physical effects. Journal of the American Institute for Conservation41(2):243–54. Sadov, F. I., and G. B.Markova. 1954. Chitosan for sizing. Tekstil'naia Promyshlennost'14(10):36. Santucci, L.1983. Insecticides and fungicides for books and documents: Treatment and effects. In Memoria del 1er seminario internacional de conservaci�n de documentos, libros y materiales gr�ficos. Mexico City: General National Archive Press. 42–57. Strnadov�, J., and M.Dur�vic. 1994. The cellulose ethers in paper conservation. Restaurator4(15):220–41. TAPPI. 1985. Zero-span breaking length of pulp, T231 cm-85. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1990. Sampling and accepting a single lot of paper, paperboard, containerboard, or related product, T400 om-90. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1993a. Fungus resistance of paper and paper-board, T487 om-93. Atlanta: Technical Association of the Pulp and Paper Industry. TAPPI. 1993b. Standard conditioning and testing atmospheres for paper, board, pulp hand-sheets, and related products, T402 om-93. Atlanta: Technical Association of the Pulp and Paper Industry. SOURCES OF MATERIALSFilter paperWhatman no. 1 Catalog number H-06648-17 Equipar, S.A. de C.V. Juan S�nchez Azcona No. 1447 Colonia del Valle, delegaci�n Benito Ju�rez Mexico, D.F. C.P. 03100 Cellulose ethersMethocel F4M Premium CMC and MC Droguer�a Cosmopolita S.A. de C.V. Av. Revoluci�n No. 1080 Col. Mixcoax Mexico City, Mexico ChitosanThe chitosan was prepared from shrimp shells by the Chemical Laboratory of the Karl Augustin RK Print-Coat Instruments Ltd. Royston Herts 5G8 OQZ U.K. SoftwareStatgraphics Statistical Graphics System Educational Institution Edition—Version 7 Manugistics Inc. and Statistical Graphics Corporation 9715 Key West Ave. Rockville, Md. 20850 Sterilization equipmentSterilization with ethylene oxide was done in regional hospital number 46 of the Social Security Institute (IMSS) Calzada L�zaro C�rdenas y 8 de Julio Zona Industrial C.P. 44940 Guadalajara, Jalisco, Mexico AUTHOR INFORMATIONMARIA DEL PILAR PONCE-JIM�NEZ received her biology degree from the National Autonomic University of Mexico and her master's degree from the University of Guadalajara with a specialty in cellulose and paper. Since 1990, she has been professor of conservation and restoration of paper and graphic documents at the National School of Conservation and Restoration and Museography at the Manuel del Castillo Negrete Institute of the National Institute of Anthropology and History (INAH) in Veracruz, Mexico. She also develops methods of teaching, application, and investigation in the field of paper conservation. Address: Centro INAH Veracruz, Benito Ju�rez No. 425 y 431, Zona Centro, C.P. 91700, Veracruz, Veracruz, Mexico. FERNANDO A. L�PEZ-DELLAMARY TORAL received his B.S. from the National Autonomic University of Mexico and his Ph.D. from the University of Wisconsin at Madison. He is a professor of chemistry and analytical chemistry at the University of Guadalajara. He is chief of the chemistry laboratory at the University of Guadalajara's Karl Augustin Grellman Institute of Wood, Cellulose, and Paper, where he heads investigative projects on paper technology, biochemistry, and forestry engineering. Address: Departamento de Madera, Celulosa y Papel Karl Augustin Grellman, Apartado Postal 52-93, C.P. 45020, Zapopan, Jalisco, Mexico. HUMBERTO GUTIERREZ-PULIDO holds a master's degree in statistics with a specialty in industrial productivity. He is a professor of design and experiment analysis in the advanced courses of the Exact Sciences and Engineering University Center at the University of Guadalajara. He does counseling, teaching, research, and applied projects in the field of statistics. Address: Centro Universitario de Ciencias Exactas e Ingenier�as, Boulevard General Marcelino Barrag�n y Calzada Ol�mpica, S. R. C. P. 44420, Guadalajara, Jalisco, Mexico.
 Section Index Section Index |