GREEN, YELLOW, AND RED PIGMENTS IN SOUTH AMERICAN PAINTING, 1610–1780ALICIA SELDES, JOS� E. BURUC�A, GABRIELA SIRACUSANO, MARTA S. MAIER, & GONZALO E. ABAD
3 RESULTS3.1 GREENSAnalyses of all green pigments indicated a copper ingredient (table 1). Electron microscopy showed crystals resembling malachite, mentioned in ancient treatises and in documents as mountain green. Chemical tests revealed the specific combinations and proportions that differentiate it from green earths. 3.1.1 Copper ResinateSix paintings were found to contain copper resinate, and these are listed in table 2. Copper resinate is a compound of cardenillo (verdigris) and a vegetable resin, similar to those obtained from some conifers or from copal trees, used as incense by Native Americans. It is composed mainly of copper salts of resinic acids. Spanish art literature refers to this resin as grassa or grasilla, also called sandarac by the Arabs. It was commercialized in Europe in liquid form, as a powder (dissolved in linseed oil to make a varnish similar to the liquid vernice of the Italians), and also in grain form (vernice in grana). It is worth mentioning that Spaniards used the green cardenillo varnish for coating or making velature on a dried base of a�il (indigo) and white, whereas it was found in some of the paintings of this collection applied directly on the primer layer, a procedure that produces an intense green. Mateo Pisarro used these recipes in his works, experimenting to obtain different hues, as exemplified by his ingenious use of copper resinate as a velatura upon an azurite blue for
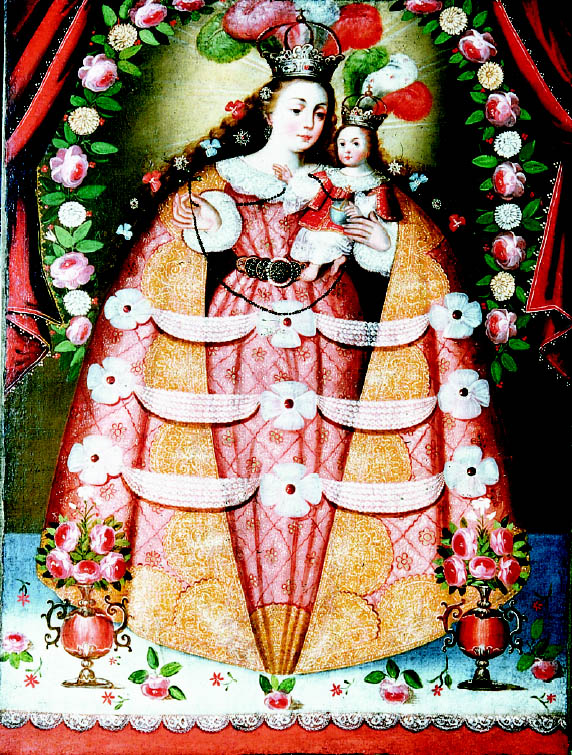
Virgin of the Rosary of Pomata, at Casabindo (painting 1.1.3 [fig. 1]), is an interesting case because the above-mentioned resin may be found mixed with a pigment whose dissolved crystals are similar in appearance to malachite rather than to cardenillo. This fact is rather striking, since it contradicts recipes from classical manuals. Although we cannot confirm the presence of malachite, it is noteworthy to find such an unorthodox procedure in the techniques of a master like Pisarro, who ventured daring and subtle technical experimentation to achieve different hues.
Cardenillo, or verdigris, is basic copper acetate, the greenish or bluish corrosion that gathers on objects made of this metal. Palomino defines it as the “beautiful green of copper rust, under the vapors of vinegar” or lemon juice ([1723] 1988, 558). Although it is extremely poisonous, it was highly appreciated for its drying qualities in oil painting. However, Pacheco advised against its use in the following terms: “I do not use cardenillo, except with thin and cheerful blue ashes.” He obtained light and dark green hues by mixing this blue pigment with g�nuli and encorca ([1649] 1990, 484). Cardenillo is also mentioned in the
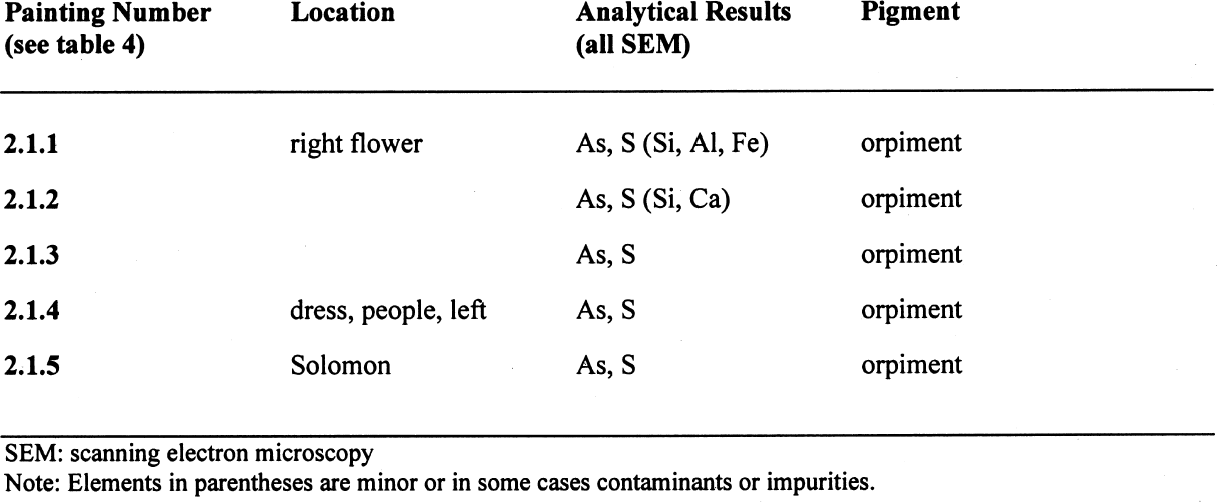
In extended areas painted with copper resinate, the original green color appeared as a faded brown layer. Such a surface-degradation phenomenon was clearly noticeable in stratigraphic cross sections, and the underlying layer had the original green color. Moreover, this alteration of the original color was absent in areas not exposed to light, i.e., in areas protected by frames or bordering tapes. It is possible that this discoloration might result from a photocatalytic reaction, specifically that such change in color may involve the photo-oxidation of the organic components of the pigment accompanied by the probable reduction of copper species to copper (I). At present, however, there is no experimental evidence to support this suggestion (Goetghebeur and Kockaert 1980–81). 3.1.2 MALACHITEThe three paintings that were found to contain malachite are listed in table 2. The malachite-based pigment, a basic copper carbonate extracted from the semiprecious stone of the same name, certainly came from the copper mines of Cerro Sapo, Bolivia (near Cochabamba), and Cazpana, Chile (province of Antofagasta), where azurite was also extracted (Seldes et al. 1999). Spanish manuals record it as mountain green or granillo, “which contributes to green hues” (Pacheco [1649]1990, 454). It was also known as green verditer, verde azzurro, Armenian stone (Montagna 1993; Roy 1993; Calvo 1997), chrysocolla, or chrysolite, according to Cobo: “A green stone that the Indians from the province of Lipes bring from their ancient mines to Potos� for sale, and is called coravari by the Indians of Peru … is none other, it seems, than the gem Dioscorides named chrysolite. Besides being useful for painters for its lovely green hues, it is of avail for many other purposes” (1890, 272). Malachite, a close relative of azurite, resulted sometimes in a greenish blue hue. Some sources refer to a blue-green color, a mixed color derived from the frequent combination of azurite and malachite in the same vein (Palomino [1723]1988, 557; Cobo 1890, 289). It seems that this color had been used from pre-Hispanic times and had—according to Father Alvaro Alonso Barba, priest at Saint Bernard's parish at the Imperial Villa of Potos�—optimum qualities for painting as well as healing powers. When referring to the colors of mineral ore extracted in the Andean area, Barba precisely described this hue as a “greenish blue” and explained that it is derived from the “Armenian stone or cibairo of the same color, and therefore painters call the color resulting from it blue-green” (Barba [1640]1967, 59). He later noted purging melancholy as among its powers. 3.1.3 Indigo and OrpimentA mixed green of indigo and orpiment was found on two paintings, which are listed in table 2. 3.1.4 Prussian Blue and OrpimentA mixed green of Prussian blue and orpiment was found on one painting, described in table 2. 3.2 YELLOWSThe results of the analyses of yellow pigments are shown in table 3. All five paintings that had yellow hues were found to contain orpiment. These paintings are listed in table 4. 3.2.1 ORPIMENTWarnings from scholars against the use of orpiment led to this pigment's gradual disappearance from the European palette, so its occurrence in our corpus is noteworthy. Also known in Spanish as jalde, orpiment
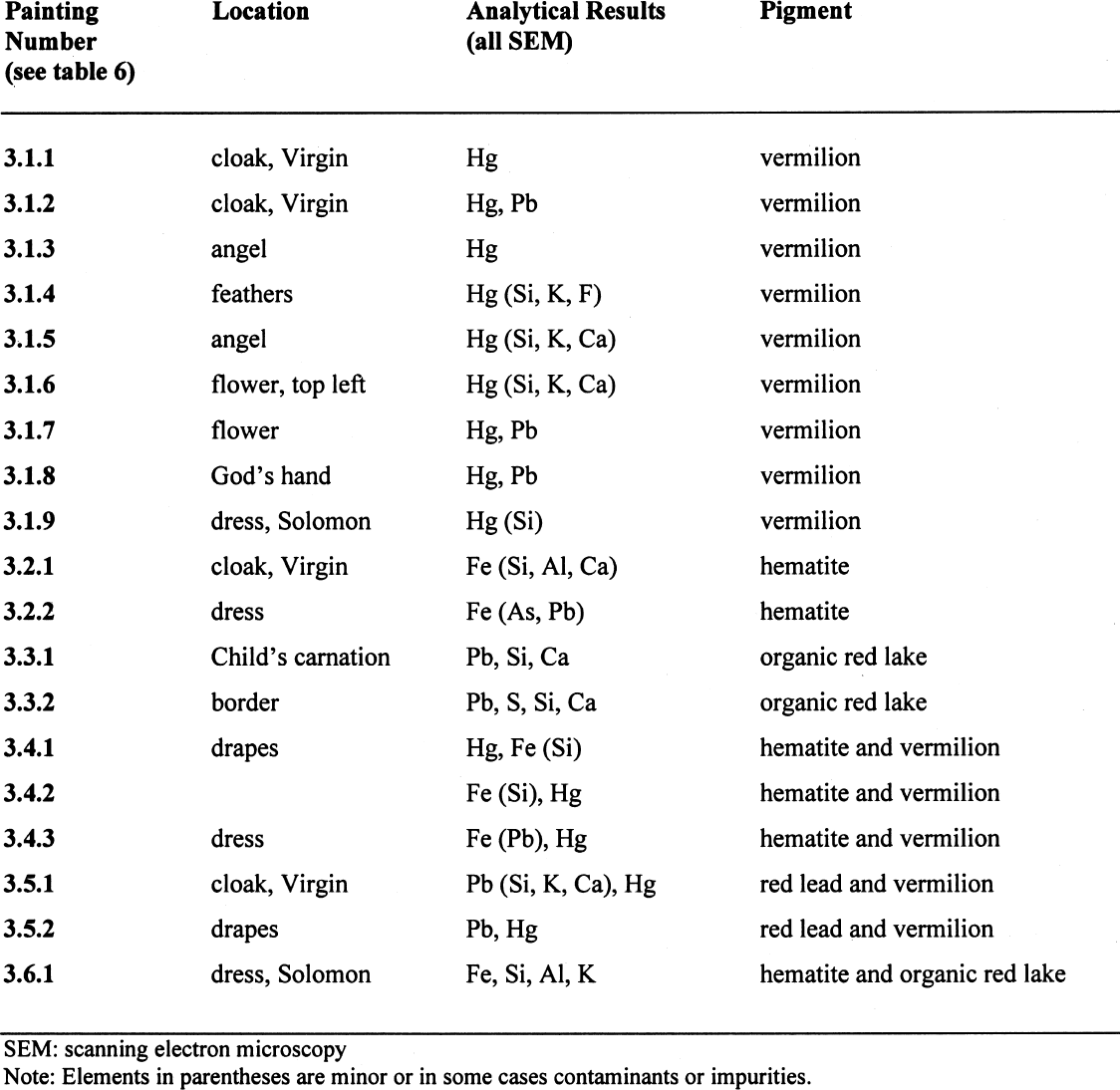
3.3 REDSBoth red and blue pigments have a prominent place within the color spectrum because of their economic and social meaning. In 15th-century Tuscany, carmine was the most expensive and highly prized pigment, even more than ultramarine. In symbology, it had the same status as purple, an expensive and highly coveted color from classical times onward, insofar as its presence in cloths suggested the dignity of kings and cardinals. The results of the analyses of red pigments are shown in table 5, and the paintings bearing red pigments are listed in table 6. 3.3.1 VermilionVermilion, also called cinnabar, is a natural mercury sulfide (HgS) extracted from quicksilver mines in Almad�n, Spain, and Idrija, Slovenia. In America, it was almost certainly extracted from Huancavelica mines (Peru, near Ayacucho), which were already exhausted in the 18th century. Cobo says that “the Indians from Peru use the name of Llimpi for quicksilver, the same metal from which they get vermilion” (1890, 322) for their paintings. Thus, the Jesuit priest Jos� de Acosta emphasized the use of this pigment called Llimpi by the Incas and natives from Peru: “it is highly prized for the same purpose Pliny has reported, … that of painting or dyeing their own faces and bodies as well as those of their idols, a very frequent practice in the past, especially in wartime, and also nowadays for feast days and dancing, which they call embijarse, because in old times they thought that thus painted their faces inspired awe, and now they feel they are decked in their best” (Acosta 1940, 251–52). Barba remarks that, before the conquest, the Indians from Potos� were not great consumers of quicksilver because the only use they made of it was to obtain cinnabar or vermilion for ritual purposes ([1640] 1967, 55). Such intense red color might be associated, then, with domination and war practices: it is also known that red was essential in the war attire of the Andean region (Molina and Albornoz 1988, 103) and was involved in rituals that were probably representations of war (as in the case of t'inku) and various sacred ceremonies. The Spanish word embijarse suggests a linguistic shift from bixa, the American name of a vegetable dye that was later adopted in botany to designate Bixa orellana or achiote (Arona 1883). Artificial vermilion was also known at the time, a vivid red color resulting from the roasted mixture of mercury and sulfur. The aforementioned documents of 1581, 1679, and 1823 explicitly cite vermilion in their list of colors. In the set of paintings under study, the fine grinding in works from Pisarro's workshop should be noted and contrasted with a coarser one in Marcos Zapata's paintings. Of the paintings in which vermillion was found, four are attributed to Mateo Pisarro or his workshop, two are from Cuzco, one is from Alto Peru, one is by Marcos Zapata, and one is probably of European origin (see table 6). 3.3.2 HematiteEarths were widely used as pigments in the Andean region. Most of them are light and dark
Hematite was found in a painting from Tom�s Cabrera and in one canvas from Cuzco, both from the 18th century (see table 6). 3.3.3 Organic Red LakeCarmine is an organic pigment, a red lake
As for South America, there are reports of wild cochineal in Loja, Ecuador; Carora, Venezuela; Tucum�n, Argentina; Peru; and Brazil, though most sources show, as in the case of a�il, a wide proliferation of the species together with a scant production and consumption. For cochineal “growing” to result in a bountiful “harvest,” a series of precautions and specific steps, which were entrusted to the Indians, were required. The whole process might be described as follows: First, nopal trees were planted, and after two or three years' growth, they were “seeded”—in other words, female cochineal insects ready for reproduction were scattered on the trees. The females were transported inside nests built with various materials and tied up to nopal stalks. Once the brood was born, they clung to the nopal tree, getting nourishment from its juices. When the new generation abandoned the nests, females were collected with soft brushes: it is from their dried bodies that the dye is obtained. When the final stage of the cycle was reached, the remaining cochineal were removed and killed using various methods—toasting, boiling, or steaming—and dried, in order to extract carminic acid, the desired dye of intense red color. The pigment itself was sent to market in loaves, in which form, to our knowledge, it reached the workshops of the artists. It was also used to immerse and ornament patterned clothes of lesser quality or cheaper price. The 1679 document cited above mentions the use of vermilion and magna. Organic red lake was found in two paintings, one by Tom�s Cabrera and the other by Marcos Zapata (see table 6). 3.3.4 Hematite and VermilionHematite appears in South American colonial sources related to vermillion in descriptions of ritual indigenous practices (Cobo 1890, 246). Paintings found to contain the mixed red of hematite and vermilion are listed in table 6. 3.3.5 Red Lead and VermilionTo obtain an orange-red color, painters used red lead, also known as minium or Saturn red, a burnt lead oxide (Pb3O4). This pigment was difficult to obtain, since it involved heating white lead under an air stream—the greater the oxidation, the darker the color—until it eventually turned bright orange. Mixed with linseed oil, red lead was used as a drier for carmine and vermilion. As mentioned above, Palomino classified red lead among “false” colors because of its tendency to shift to duller hues as the paint dried. However, this dulling could be prevented by purification with a vinegar washing. Pablo de C�spedes, according to a quotation found in Pacheco's work, ranked it among the “florid” and very expensive tempera paints and called it vermilion, a clear indication of the terminological confusion of the times with respect to these colors and many others ([1649] 1990, 445). In the Art of Metals, Barba himself considers red lead and vermilion the same mineral ([1640] 1967, 59). There are references to this pigment in the 1581, 1679, 1767, and 1823 contracts mentioned above. The mixture of red lead and vermilion was found in a painting from Mateo Pisarro and in another from Marcos Zapata (see table 6). 3.3.6 Hematite and Organic Red LakePaintings containing mixed reds of hematite and red lake are listed in table 6. |