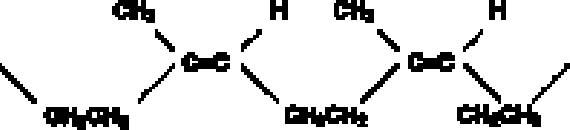A CASE STUDY IN THE USE OF CYCLODODECANE AND LATEX RUBBER IN THE MOLDING OF MARBLEJEFFREY P. MAISH, & ERIK RISSER
ABSTRACT—The authenticity of a Roman marble bust of a pugilist was questioned on technical grounds because of inconsistencies in carving. Tool markings suggested the head had been joined to the torso either as an ancient reuse or a modern pastiche. But translucency and variation in the marble coloration made tool markings difficult to interpret. A plaster cast of the original, however, with its uniform white and opaque surface might make these markings more visible. Materials selected for the molding process must not stain the marble, and no unbound, modern pigment should be retained on the marble. At the same time, no isolating barriers could be applied, as the surface rendering of the cast had to be of sufficient clarity to preserve fine details. In addition, the mold should not remove loosely bound material. This article describes the process of selecting materials that could work within these constraints. Latex rubber, a molding material used for the past century, was employed in conjunction with cyclododecane to meet these criteria. TITRE—Une �tude de cas sur l'utilisation du cyclodod�cane et du latex en caoutchouc pour le moulage du marbre. R�SUM�—L'authenticit� d'un buste romain en marbre d'un pugiliste a �t� mise en question en raison d'incoh�rences not�es dans la technique de taillage. Les marques d'outil sugg�raient en effet que la t�te avait �t� jointe au torse, soit lors d'une r�utilisation dans l'Antiquit� ou comme r�sultat d'un pastiche r�cent. Mais la translucidit� du marbre et les variations de sa couleur rendaient ces marques d'outils difficiles � interpr�ter. Toutefois on �tait d'avis qu'un moulage en pl�tre fait � partir de l'original pouvait rendre ces marques plus visibles, de par la surface uniforme blanche et opaque du pl�tre. Les mat�riaux choisis pour le processus de moulage ne devaient pas souiller le marbre, ni aucun colorant moderne ne devait �tre retenu sur la surface du marbre apr�s l'op�ration. En m�me temps, aucune couche isolante ne pouvait �tre appliqu�e sur l'original, car les fins d�tails de la surface risquaient d'�tre estomp�s sur la copie. Enfin la surface du marbre �tant fragile, le processus de moulage ne devait provoquer aucun d�tachement de fragment ou de grain de mati�re. Cet article d�crit le processus de s�lection de mat�riaux qui pouvaient �tre utilis�s malgr� ces contraintes. Le latex en caoutchouc, un produit de moulage employ� depuis plus d'un si�cle, utilis� en association avec du cyclodod�cane, a pu r�pondre � ces crit�res. TITULO—Un estudio de caso acerca del uso de ciclododecano y goma l�tex para el moldeado de m�rmol. RESUMEN—La autenticidad de un busto romano de m�rmol de un pugilista fue cuestionada sobre bases t�cnicas debido a inconsistencias en el tallado. Las marcas de herramienta suger�an que la cabeza habr�a sido unida al torso, ya sea como una reutilizaci�n antigua, o como un pastiche moderno. Pero la traslucidez y la variaci�n en la coloraci�n del m�rmol hacen que las marcas de las herramientas sean dif�ciles de interpretar. Un molde de yeso del original, con su superficie uniformemente blanca y opaca, podr�a hacer estas marcas m�s visibles. Sin embargo, los materiales a seleccionar para el proceso de moldeado pueden manchar el m�rmol, y los pigmentos modernos sueltos no pueden quedar retenidos en el m�rmol. Asimismo, no se podr�n aplicar barreras de aislamiento ya que la superficie terminada deber� tener claridad suficiente como para preservar los detalles finos. Adem�s, el molde no debe remover el material fr�gil de la escultura original. Este art�culo describe el proceso de selecci�n de materiales que podr�an funcionar con estas limitaciones. La goma l�tex, un material de moldeado utilizado durante el siglo pasado, fue empleada en conjunto con ciclododecano para reunir estas condiciones. 1 INTRODUCTIONThe technical examination of a marble bust of a pugilist (fig. 1) in the collection of the J. Paul Getty Museum, Los Angeles, California, raised questions regarding the relationship of the head to the body.
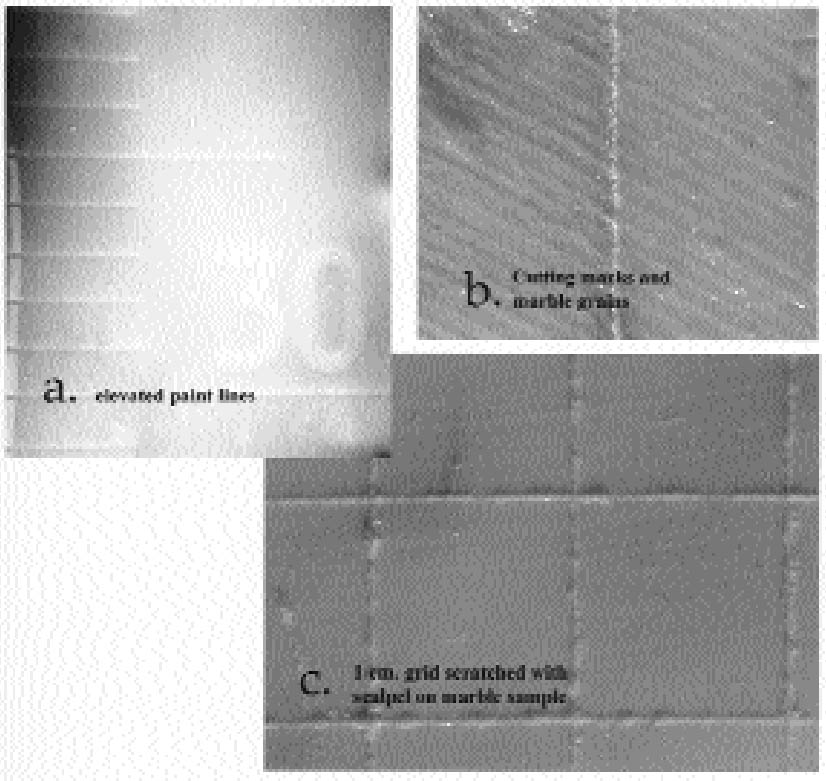
2 MATCHING BARRIER TO MOLD MATERIAL2.1 RUBBERIn mold making, conservators have long been aware of the possibility of transferring mold-associated materials to the surfaces of art objects. The use of a protective barrier in itself presents challenges in that this material must be subsequently removed and in instances may interfere with the molding itself. A variety of molding materials are available, but each has characteristic problems. Polyurethane rubbers can achieve good detail but bond tenaciously to unprepared substrates. Polysulphide rubbers are less expensive, but they produce substantial oily stains. Water-based alginates can render good detail in small molds but lack the strength for larger-scale molds. Silicone rubbers applied without barriers are known to transfer a silicone oil that in general must be removed by poulticing (Maish 1994). Considering the near life size of the marble bust, room temperature vulcanization rubber (RTV 2) was initially considered, but it would require the application of some type of protective barrier to the marble. Trials with different rubber types revealed that a high-solids latex left a test marble surface without a barrier unaltered and, in this particular case, had good releasing properties. Under low-power magnification the latex rubber provided visual detail approximating that of a silicone rubber RTV 2 (fig. 2). In one published case, sufficient detail was achieved for making detailed replicas for scientific research (Baird 1965).
The disadvantages of latex rubber include extreme shrinkage due to loss of water upon drying and short mold life. Small-scale tests suggested that latex renders a high level of detail and that most if not all shrinkage in the rubber occurs orthogonally, or perpendicular to the surface being molded. Since shrinkage is not occurring parallel to the surface, dimensions are rendered fairly faithfully. However, because latex requires air-drying, only thin coats can be applied at a time. Ammonia is often present in latex rubbers and can have deleterious effects on some materials, especially metal alloys, as it can leach components and artificially enrich a surface. Latex rubber molds have a relatively short life and would be of use only if a set of casts were produced shortly after mold production. Natural rubbers, in general, have a high proportion of double bonds, making them especially susceptible to oxidation, with disproportionate effects to physical properties (Mills and White 1987)(fig. 3). In this study, however, use of a water-based rubber was considered an advantage when used in conjunction with a nonpolar barrier. Tests with a high-solids (74%), no-ammonia latex (Latex 74, A-R Products) were encouraging. Latex rubber air-dries in fairly thin layers, and, traditionally, multiple layers of the material are applied and partly reinforced with gauze until sufficient mold thickness is achieved. This procedure may increase production time, and to expedite this thickening process, several trials were conducted to gauge whether an RTV rubber could be used to support or back a thinner layer of latex rubber. The ability of an RTV rubber to set in a thicker layer would, we hoped, shorten work time, help create a uniform rubber thickness, and provide a better fit to a resin mother mold. Tests were conducted to gauge whether latex could be bonded to either a silicone or nonsilcone type RTV rubber, and a urethane (Ever-green 30A) was selected to take advantage of its bonding properties and slight adhesion to latex.
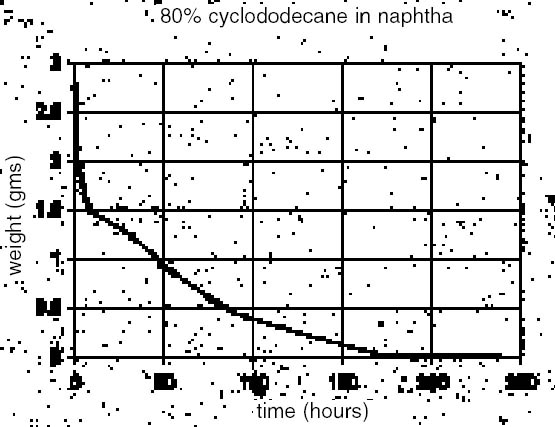
2.2 THE BARRIERSome success has been reported with silicone rubber used in conjunction with certain barrier types. Pulga (1997) reports on a peelable barrier, while Br�ckle et al. (1999) applied a volatile/subliming cyclododecane (CDD) barrier to isolate a second waterborne barrier. The aim in the latter case was to reduce the penetration of the water-based material into the substrate and to facilitate subsequent barrier removal. CDD alone is soluble, to a degree, in silicone oils, offering little if any protection from penetrating oils during silicone rubber molding. Further testing of silicone rubber (RTV 2) at different set times on unbound pigment mock-ups also showed significant pigment loss with molding. Because of its intrinsic and promising sublimation properties, further trials were conducted to develop a methodology using CDD for molding the marble bust (see Stein et al. 2000 for properties). Cyclododecane, a waxy nonpolar material (C12H24), could conceivably work well in conjunction with a polar molding compound that would include water-based materials such as rubber latex. 3 APPLICATION OF CYCLODODECANETrials conducted with cyclododecane posed two principal technical challenges: the controlled application of a uniform layer on a large area, and the control of its property of forming a crystalline structure during solvent loss or cooling. The application of CDD in solid form can be a slow process, producing a thick and inconsistent layer with unwanted crystalline features. Considering the ultimate goal of studying tool markings, these effects were deemed a hindrance. Solvent application of CDD was investigated as an alternate method of application and means of crystallization control, but it was observed to have an additional adverse film-disrupting effect on the latex rubber, most probably due to the solvent. 3.1 SURFACE APPLICATION PRACTICALITIES OF CYCLODODECANETo maximize the CDD concentration without obscuring surface details, the authors initially applied molten CDD to a test surface with the further aid of a heat gun. This method, although perhaps suitable for small areas, was also proved unsuitable for providing a uniform surface free of artifacts that would be captured in subsequent molding. Further trials were conducted with CDD in two recommended solvents: odorless mineral spirits and naphtha (Hangleiter 2000).1 Solubility tests indicated that high concentrations could be achieved with both, but the authors obtained higher concentrations in naphtha (95% w/v in naphtha vs. 65% w/v in odorless mineral spirits). Concentrations as high as 140% in hexanes have been reported (Stein et al. 2000). The main limitation to these higher concentrations appeared to be the more rapid crystal formation that could obscure original surface detail. Application of a slightly warmed and less-concentrated solution overcame this limitation, providing a more controllable application method that slightly penetrated the stone surface while yielding a more uniform layer. Rapid cooling (Hangleiter 2000) has reportedly been used to reduce crystal size, but in this instance, it is speculated that the surface morphology of the stone inhibited the crystal formation to a degree. A weight-loss trial with 80% CDD in naphtha (fig. 4) shows a high initial rate of mass loss that would presumably correspond to solvent evaporation (naphtha) with a subsequent slower loss rate reflecting the CDD sublimation, perhaps in combination with some remaining solvent. The graph implies that CDD could be deposited on the surface and latex applied following an initial solvent loss. 3.2 DEVELOPING A TIMING WINDOW: THE SOLVENT-LATEX INTERACTIONThe solvents in the CDD mixes appeared to cause an uneven drying and buckling of the latex rubber. Because of this observed deleterious solvent
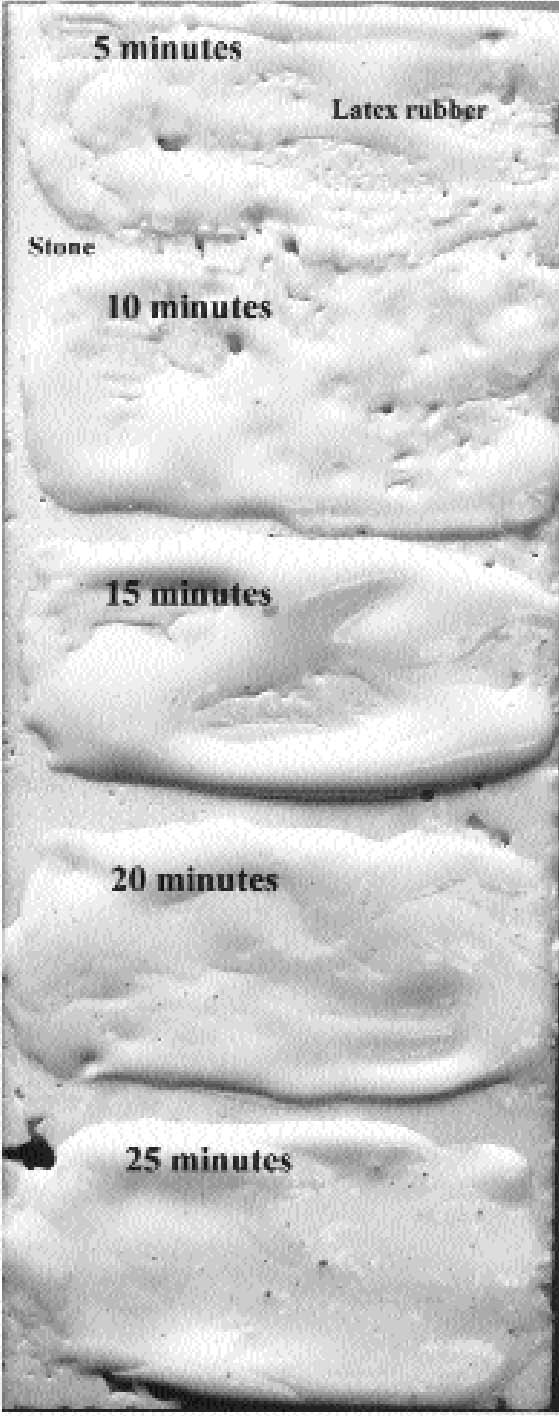
CDD (20% in odorless mineral spirits) was applied to the entire test surface, and latex was brushed on in regular strips at five-minute intervals up to 25 minutes during the CDD–solvent-drying process (fig. 5). The odorless mineral spirits solvent was observed to have considerable adverse effect on the latex before 10 minutes of solvent evaporation time. By 25 minutes, the CDD had developed a quite prominent and visible crystalline pattern. The ideal window was therefore judged to begin in the vicinity of 20 minutes after the application of the CDD, when excess solvent had evaporated but cyclodode-cane still remained as an undifferentiated barrier. At this point, latex rubber could be brushed on, and, once dry, the rubber served not only to mold the first layer of detail on the surface but also as a barrier to succeeding applications of materials. In effect, the CDD formed a barrier to this first coating of latex rubber.
3.3 CRYSTAL FORMATION AND BARRIER PROPERTIESTo better understand CDD crystalline growth, incremental 20% w/v (20%, 40%, 60%, 80%) solutions of CDD in naphtha and odorless mineral spirits were prepared. Solutions were warmed to 30�C, and a single, thin brush stroke was applied to standard microscope slides to aid in visualization and interpretation. To better understand the sublimation processes, digital time-lapse photomicrography (using a Kodak MDS 100 camera) was used to observe the surfaces over a four-hour period. Similar results were observed for both solvents, with higher concentrations of CDD in solvent producing smaller crystals but forming crystals more rapidly. Naphtha appeared to produce slightly smaller crystals than odorless mineral spirits. Higher concentrations (i.e., with a greater relative amount of CDD) also produced fewer visible holes toward the end of the sublimation period (a period of hours), and the barrier layer took longer to sublime. After four hours on the glass slide, all but the 80% solution in odorless mineral spirits appeared to have sublimed completely, although faint tide lines were visible on some slides. 3.4 RESIDUESFollowing the previously mentioned weight trial using 80% CDD in naphtha (w/v), a final stage was reached where 0.02% of the starting solution weight remained unsublimed for almost a week before the weight finally returned to zero. Preliminary reports (Stein et al. 2000; Caspi and Kaplan 2001) have suggested that some residue is left by CDD, and this was confirmed in this trial. Even though the balance measured to an accuracy of 0.0001 gram and eventually returned to 0 weight, a faint “tidal” film could still be observed on the watch glass. At this point the nature of the film is undetermined, but it is likely from the CDD as no residue was noted for the evaporation of naphtha alone. In either case, the amount was undetectable by fairly precise weighing alone and would require further analytical study. 3.5 MOLDING EFFECT ON UNBOUND PIGMENTThe final parameter of the CDD test was to gauge its effectiveness in protecting a loosely bound material during molding. Latex rubber alone was determined to have no visible effect on unprotected marble, and CDD was therefore to be applied only in areas containing unbound pigment. A comparison was made to gauge the protective effects of 20%, 40%, 60% and 80% CDD solutions in odorless mineral spirits and naphtha on an unbound pigment layer (fig. 6). To maximize the deposition of dry pigment, a porous travertine marble sample was brushed with terra rossa dry pigment and distributed by wiping over the stone's surface. The warmed CDD solutions of varying concentrations were gently brushed onto the surface in two sets of four rectangles, and after 20 minutes' solvent evaporation time, high solids latex rubber was applied over all test rectangles and allowed to dry. The latex was then peeled back, revealing the underlying test patches. The 20% CDD solutions in both odorless mineral spirits and naphtha provided virtually no protective effect to the pigment. At 80% concentration in naphtha, however, it is estimated that roughly 10% of loosely bound pigment was picked up, whereas, unexpectedly, half of the pigment was picked up in the adjoining 80% cyclododecane in odorless mineral spirits square. The solution in naphtha appeared to perform substantially better than the solution in odorless mineral spirits in inhibiting pigment removal. This finding suggests that solvent choice may play a critical role in protecting unbound pigment. In tests on glass slides, CDD in naphtha crystallized more rapidly than in odorless mineral spirits, and perhaps this CDD-naphtha combination produces a better protective layer. The solvent itself (odorless mineral spirits) may also have had a more mobilizing effect on unbound pigment. In light of all the trials, and to maximize the concentration of CDD, a 90% solution (w/v) in naphtha was selected as a barrier to be used in conjunction with latex rubber. Additionally, when warmed slightly on a hot plate to 30–40�C, this solution flowed quite evenly.
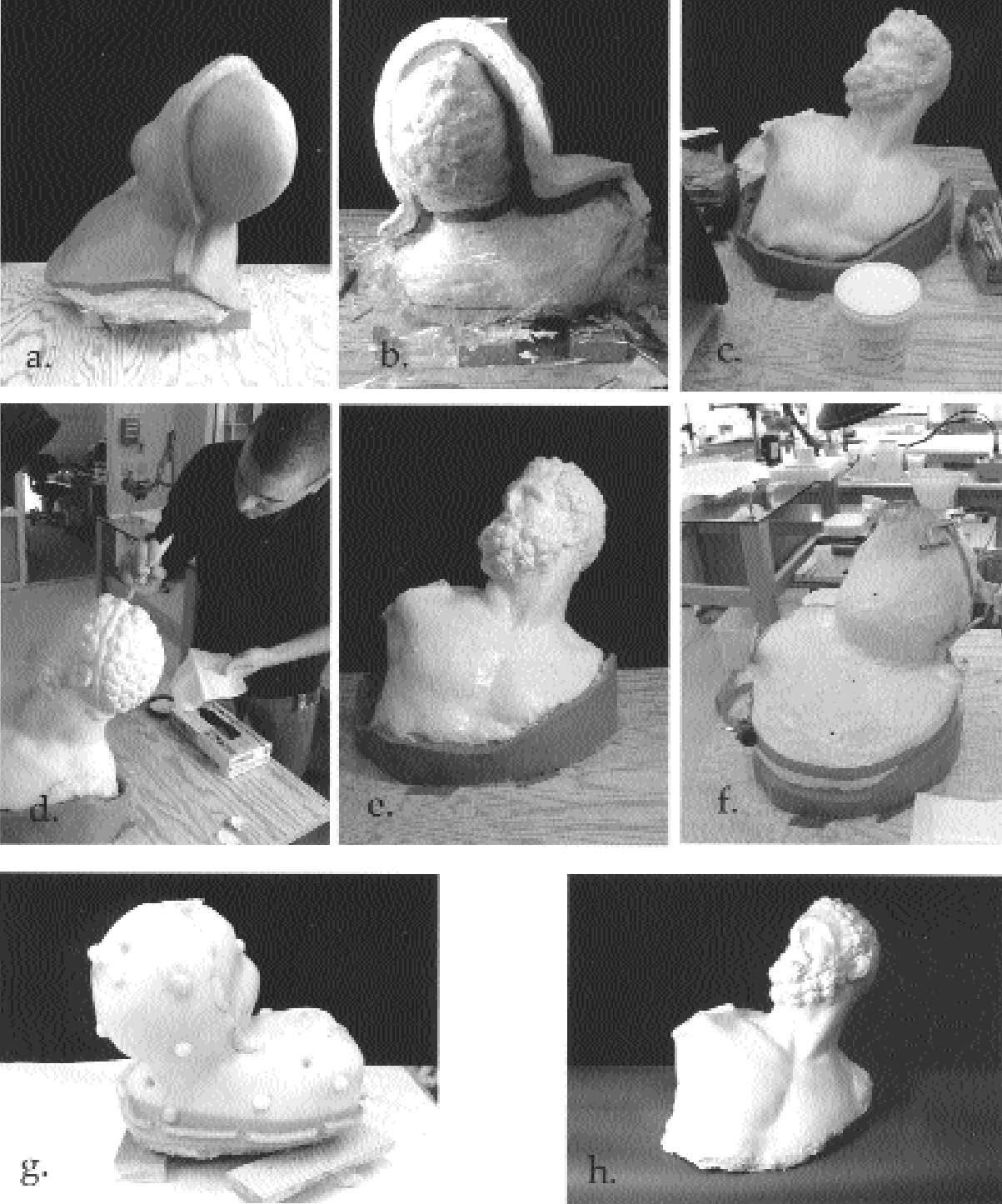
4 THE MOLDMolding of the bust was done in the traditional manner, by building a clay thickness over the isolated object (plastic wrap) and casting the mother mold first (fig. 7). The mother mold and clay were then removed. Following preparation, the warmed CDD in naphtha was brushed over the pigmented marble surfaces, and the solvent was allowed to evaporate for 20 minutes. Unpigmented areas of marble were not coated in order to achieve maximum detail in the mold. Latex rubber was then brushed onto the entire bust and gradually built up in layers. The mother mold was reintroduced to the piece, and urethane rubber was poured to fill the final void between the latex rubber and mother mold. Once set, the mother mold was removed, and the multiple-layer rubber latex–urethane mold was carefully sliced and removed from the bust. 5 DISCUSSIONAlthough somewhat time-consuming, the mold achieved very good detail without staining. The consolidation of “pigmented” areas with CDD was mostly effective. The modern yellow toning remained in the beard, although some yellow toning in the hair appeared to have been reduced. There was additional movement of water-soluble grime caused by the latex in areas of the marble that had been purposefully left uncoated with CDD. There was no surface adhesion (latex to stone), although a few grains of marble were pulled from an abraded, sugary, and unconsolidated area on the rear of the bust. The rubber latex–cyclododecane combination produced very good detail, and the plaster cast was of sufficient quality to aid in the technical study of the bust of the pugilist. Although silicone molds provide a more straightforward method of molding objects, more time may be needed for postcast cleanup and removal of barrier coatings, and barriers may never completely come out of porous substrates. The timing of the solvent evaporation and the mutual repellence of the waxy (nonpolar) CDD and water-based (polar) latex rubber was an advantage. CDD appears to leave almost immeasurable residue amounts that would benefit by further characterization (the authors plan to submit samples for identification). Ideally, molds will last indefinitely as archival documents, and the longevity of this latex-polyurethane mold is to be determined, although it is expected to be much less durable than silicone rubber. The lack of longevity for latex is due to its double-bonded chemical structure, which makes it susceptible to oxidation. Perhaps this type of mold could be stored with a final plaster cast in situ or in an anoxic bag to preserve dimensional stability and/or retard oxidation. Finally, although the tool markings were not being studied by electron microscopy, a silicone rubber could be expected to perform better at this higher magnification. 6 CONCLUSIONEach mold-making process presents very unique challenges. In a visual comparison, the latex rubber mold and plaster cast achieved excellent detail and aided in the subsequent technical study. The selected rubber latex used in conjunction with a cyclodode-cane barrier had minimal surface effects in protected areas, and the latex itself affected only water-soluble soil components on the stone surface. This combination of new (cyclododecane) and improved old (ammonia-free latex rubber) materials and methods shows some promise, although any future molding attempts should always be preceded by careful testing. Unfortunately, latex rubber is inherently unstable, but mold life might be prolonged by careful storage planning. The question of cyclododecane residues remains. Research to date suggests the amounts are extremely small and would be detectable or at least identifiable only by more sophisticated analytical methods. In any case, the amount of residue left by cyclododecane is minimal in comparison to residue amounts left following the removal of resin barriers.
ACKNOWLEDGEMENTSThe authors would like to acknowledge Jerry C. Podany, head of the Antiquities Conservation Department at the J. Paul Getty Museum, for presenting us with this molding project and its challenges. NOTES1. Safety and ventilation precautions should be observed when handling these materials. Additional caution should always be exercised in heating any mixture containing a solvent. Cyclododecane melts at 61�C and has a flash point of 95�C. Naphtha has a flash point of 50�C. Cyclododecane is considered an irritant. REFERENCESBaird, D.1965. Latex micro-molding and latex-plaster molding mixture. Science122:202 Br�ckle, I., J.Thornton, K.Nichols, and G.Strickler. 1999. Cyclododecane: Technical note on some uses in paper and objects conservation. Journal of the American Institute for Conservation38:162–75. Buttle, J. W., D. J.Daniels, and P. J.Beckett. 1974. Chemistry: A unified approach,. London: Butterworths. 369. Caspi, S., and E.Kaplan. 2001. Dilemmas in transporting unstable ceramics: A look at cyclododecane. Paper presented at Objects Group Session, AIC 29th Annual Meeting, Dallas, Tex. Hangleiter, H.2000. Cyclododecane (CCD) eMaterialinformation. www.hangleiter.com (accessed May 2002). Maish, J. P.1994. Silicone rubber staining of terracotta surfaces. Studies in Conservation39:250–56. Mills, J., and R.White. 1987. The organic chemistry of museum objects. London: Butterworths. 99–101. Pulga, S.1997. A note on the use of silicone rubber facings in the reassembly of archaeological painted plasters. Studies in Conservation42:38–42. Stein, R., J.Kimmel, M.Marincola, and F.Klemm. 2000. Observations on cyclododecane as a temporary consolidant for stone. Journal of the American Institute for Conservation39:355–69. FURTHER READINGKremerPigment. 2001. Material safety data sheet 87100, cyclododecane. New York, N. Y. SOURCES OF MATERIALSCyclododecaneKremer Pigment Inc. 228 Elizabeth St. New York, N.Y. 10012 Digital Microscope CameraEdmund Industrial Optics 101 East Gloucester Pike Barrington, N.J. 08007 Latex 74A-R Products, Inc. 11807 7/8 Slauson Ave. Santa Fe Springs, Calif. 90670 Heraeus Kulzer 0–67 Snow White Plaster (.14% expansion)Patterson Dental Supply 1031 Mendota Heights Rd. St. Paul, Minn. 55120 Trowelable plastic material (mother mold), Evergreen 30A urethaneSmooth-on, Inc. 2000 St. John St. Easton, Pa. 18042 AUTHOR INFORMATIONJEFFREY P. MAISH is an associate conservator of antiquities at the J. Paul Getty Museum and has an ongoing interest in issues concerning the replication of museum objects. He has also been actively involved in the radiography of objects, planning for radiography, and the implementation of different radiographic methods. He has participated in the technical investigation of many ancient bronzes as well as other material types in the museum's collection. Address: Antiquities Conservation Department, J. Paul Getty Museum, 1200 Getty Center Dr., Suite 1000, Los Angeles, Calif. 90049. ERIK RISSER holds a B.A. in classical archaeology from the University of Evansville and an M.Sc. in conservation for archaeology and museums from the Institute of Archaeology, University College, London. He is currently an assistant conservator of antiquities on contract at the J. Paul Getty Museum. He has previously worked for the British Museum and Institute of Archaeology on the 'Ain Ghazal Statue Project and has undertaken and directed conservation activities on archaeological sites in Italy, France, and Turkey. In addition, he has worked on several mold-making projects, most recently on the large-scale molding and casting of a monumental figurative frieze in Turkey. Address as for Maish.
 Section Index Section Index |