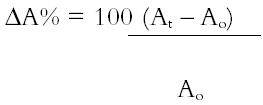THE SWELLING OF ARTISTS' PAINTS IN ORGANIC SOLVENTS. PART 1, A SIMPLE METHOD FOR MEASURING THE IN-PLANE SWELLING OF UNSUPPORTED PAINT FILMSALAN PHENIX
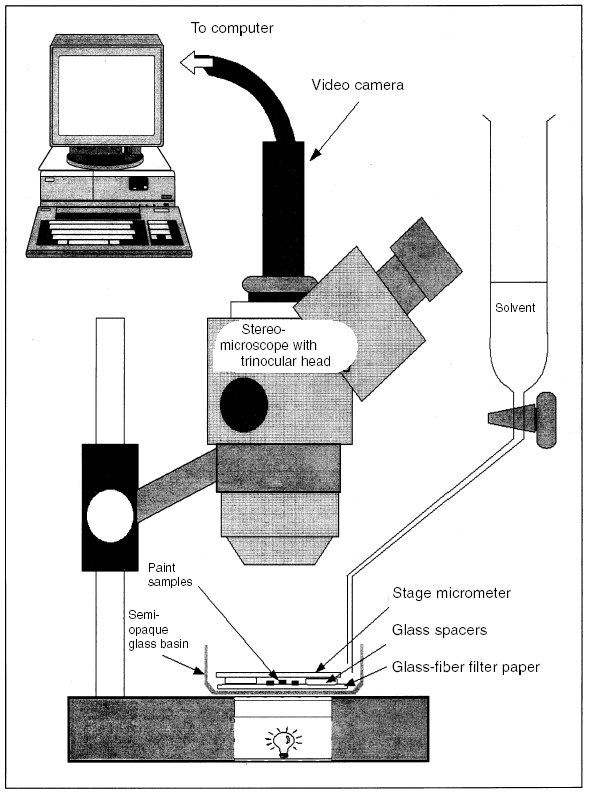
ABSTRACT—A simple method is presented for measuring the in-plane swelling of paint films when immersed in solvents. The method is a development of a low-power microscopy approach first described in the 1960s, with improvements to the measurement aspect through the use of computer-based digital image analysis. Small fragments of paint (2–5 mm2) from stock films of uniform thickness are immersed in the test solvent, and images of the samples are captured electronically at intervals from t = 0 until equilibrium swelling is reached. Image analysis software is used to measure change in the areas of the fragments over time, resulting in swelling curves of time vs. % change in area. The experimental method is described in detail, together with a discussion of key factors relevant to uncertainty in the results. The swelling behavior of various paint films in ethanol and in some other common solvents is described. TITRE—Le gonflement des peintures d'artistes dans les solvants organiques. 1�re partie: une m�thode simple pour mesurer le gonflement dans la plan pour les films de peinture non soutenus. R�SUM�—Une m�thode simple est pr�sent�e pour mesurer le gonflement dans le plan pour les films de peinture lorsqu'ils sont immerg�s dans des solvants. Cette m�thode est bas�e sur l'utilisation d'un microscope � faible grossissement, tel que d�crite dans les ann�es 60, mais avec une fa�on am�lior�e pour obtenir des mesures gr�ce � l'analyse d'images num�riques sur ordinateur. Des petits fragments de peinture mesurant entre 2 et 5 mm2 et obtenus � partir de films d'�paisseur uniforme sont immerg�s dans le solvant choisi et des images des �chantillons sont captur�es �lectroniquement � intervalles r�guliers jusqu'� ce qu'un �quilibre soit atteint. Un logiciel d'analyse d'images est employ� pour mesurer les changements progressifs dans la dimension des fragments, ayant pour r�sultat des courbes de gonflement des films comparant le temps �coul� au pourcentage de changement de dimension. La m�thode exp�rimentale est d�crite en d�tails, ainsi que des facteurs importants concernant une certaine incertitude dans les r�sultats. Le gonflement particulier de plusieurs films de peinture dans l'�thanol et dans quelques autres solvants couramment utilis�s est d�crit. TITULO—Dilataci�n en solventes org�nicos de pigmentos para artista, Parte 1: Un m�todo simple para medir la dilataci�n planar de pel�culas de pintura sin soporte. RESUMEN—Se presenta un m�todo simple para medir la dilataci�n planar de pel�culas de pintura cuando son sumergidas en solventes. Este m�todo surgi� de una t�cnica de microscop�a de bajo poder descrita por primera vez en los a�os 60, la que fue mejorada en los aspectos de medici�n por medio del an�lisis computarizado de im�genes digitales. Fragmentos peque�os de pel�culas de pintura de 2–5 mm2 de grosor uniforme son sumergidas en el solvente de prueba. Las im�genes electr�nicas son capturadas a intervalos de t = 0 hasta alcanzar el equilibrio de dilataci�n de las pel�culas. Para medir los cambios que se producen en las �reas de los fragmentos a trav�s del tiempo, se utilizan programas computarizados de an�lisis de im�genes. Estos dan como resultado curvas de tiempo versus porcentaje de cambio por �rea. En este trabajo se describe en detalle el m�todo experimental y se discuten factores claves que son relevantes a la incertidumbre de los resultados obtenidos. Se describe el comportamiento de dilataci�n de varias pel�culas de pintura en etanol y en otros solventes comunes. 1 INTRODUCTION: MEASURING THE SWELLING OF PAINT AND POLYMER FILMS DUE TO SOLVENT SORPTIONIn the removal of varnishes and overpaint from pictures using organic solvents, one of the key elements of risk to the original paint comes from the possibility that it will be swollen due to sorption of the cleaning solvent. In the swollen, gelled condition, the pigment binding power of the paint is diminished and the paint is vulnerable to loss of pigment from the mechanical action of the swab delivering the There have, over the years, been many approaches to the measurement of swelling of paint films and polymers (Thiessen 1925; Faraday Society 1946). These approaches generally fall into two categories: determination of dimension change either in the plane of the film or in the perpendicular direction; and measurement of weight gain due to solvent sorption. Weight gain is perhaps the most widely used of all methods to determine swelling of paint (Rinse and Wiebols 1937; Browne 1956; Brunt 1964; Long et al. 1967). This method—or its inverse, measurement of weight loss from material saturated with solvent—has been applied to measure swelling of many other materials in addition to paint, mostly polymer networks of various types (Barr-Howell and Peppas 1985; Errede 1992; Byun et al. 1996), including poly (vinyl chloride) (Parker and Ranney 1995, 1996) and rubber (Gee 1942; Salomon and van Amerongen 1947; Scott and Magat 1949; Bristow and Watson 1958), as well as photographic gelatin (Johnsen 1996). Degree of swelling, for example, is accepted as one indicator of the solvent barrier properties of rubbers used in protective gloves (Zellers et al. 1996a). It is common in studies of polymer swelling for the results to be interpreted using various solubility parameter treatments, of which the Hildebrand solubility parameter, ∂ (Bristow and Watson 1958; Mangaraj 1963; Stolow 1963), the Hansen solubility parameters, ∂d, ∂p, ∂h (Zellers et al. 1996b), and the Flory-Huggins interaction parameter, χ (Huglin and Pass 1968; Peppas and Merrill 1976; Parker and Ranney 1995), are the most common. The main problem associated with the gravimetric approach to measuring swelling is that samples must be removed from the swelling liquid and handled at intervals, and reliability is strongly affected by the presence in the subject material of a significant soluble phase, as in the case of oil paints. Consequently, there has been considerable interest in noncontact methods for measuring swelling in terms of change in sample dimensions. Stolow's method (Stolow 1954) was itself a development of that devised by Lewis and Soper (1950) for measurement of the swelling of gelatin films. The principle common to these two methods is that the swelling liquid impinges on the sample surface from a jet. As the sample swells or contracts, the jet is retracted or advanced to maintain a constant fluid pressure. Calibrating the movement of the jet tip provides a measure of the change in sample thickness over time without actual contact with the surface. The “swellmeter” of Green and Levenson (1972) was also developed for measuring perpendicular swelling of gelatin films, but this is a contact method: a solid feeler probe connected to a solenoid transducer measures the displacement of the probe electrically as the sample expands or contracts. Compression of the sample due to the force of the feeler probe is minimized by using a broad-faced probe and low contact time (0.25 seconds). This type of apparatus has been used within the field of conservation to measure the swelling of gelatin photographic film emulsions exposed to atmospheric pollutants (Nguyen et al. 1999). Recently, various other high-sensitivity noncontact methods have been proposed for measuring swelling of polymer films due to solvent sorption from liquid or vapor. These include ellipsometry (Chen et al. 1999; Parbhoo et al. 2000), quartz crystal microbalance (QCM) (Chen et al. 1999), and x-ray or neutron reflectivity (Tan et al. 1998). The ellipsometry method, which relies on reflection of polarized light from the boundary interfaces of the films, is capable of subnanometer resolution. It is not, however, suited to the measurement of opaque, pigmented films due to light scattering. The usual alternative approach to noncontact measurement is some form of microscopical measurement, often with a traveling microscope. This method has been applied to the cross sectional swelling of various textile fibers by atmospheric humidity (Morehead 1952). A microscopical method was used by Browne to measure paint film swelling as a complement to weight gain measurements (Browne 1956). During the 1960s, further studies appeared Michalski's compilation of paint swelling data from various sources (Michalski 1990) demonstrated good correlation between swelling results on oil paints obtained by different experimental methods. Interestingly, the results obtained by the relatively simple methods of Browne (1956) and of Eissler and Princen (1968, 1970) compare favorably with those of the more sophisticated, higher-sensitivity method of Stolow (1954, 1955). 2 EXPERIMENTAL METHODThe method for measuring paint swelling proposed here is a relatively low sensitivity technique suitable for use on unsupported paint films. Lateral, in-plane swelling is determined by a simple method that is loosely based on that of Brunt (1964) and Eissler and Princen (1968, 1970) but with significant improvement to the measurement aspect through the use of computer-based image analysis. The general experimental setup is illustrated in figure 1. Images of the paint samples during immersion in solvent are captured using a video camera fitted without lenses, via a trinocular viewing head, to a low-power stereo zoom microscope (objective range 1–4x). The video camera is linked directly to a computer: images are captured and digitized by an internal image grabber (Neotech). Two video cameras were used for the results reported here: a Moritex Micro-Scopeman MS-500HR-RGB and a JVC TK-C1380E. Both have 752 x 582 pixel array detectors. The computer used was an Apple Power Macintosh G3 computer (233 MHz, 4 GB disk, 128 MB RAM, 6 MB SGRAM, 20 in. Sony Trinitron monitor). The digitized images are in monochrome, 8-bit gray scale, i.e., a maximum palette of 256 levels of gray. All raw and processed images are archived onto a rewritable disc.
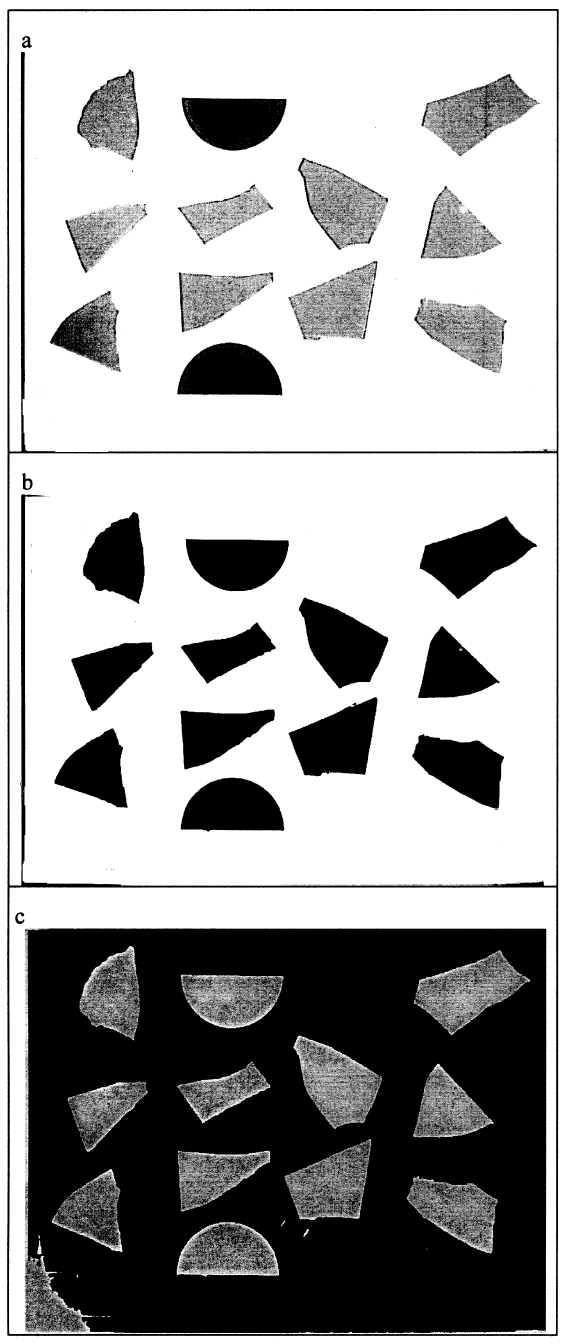
The paint fragments for testing are cut from larger samples of known thickness and are placed on a disc of glass-fiber filter paper. In turn, this disc is placed in a semi-opaque glass basin in which the swelling test is conducted. The basin is viewed through the microscope in transmitted light using the microscope's internal tungsten-halogen light source, the semi-opaque basin and filter paper serving as diffusing screens so that the paint fragments are seen as silhouettes against a uniform, bright background. To limit heat gain from the tungsten-halogen light To conduct a swelling experiment, the selected solvent is quickly run into the basin from a dropping funnel. Care must be taken not to displace the paint fragments from the field of view; this step can be difficult with solvents having high surface tension, such as aqueous solutions. The first image, representing t = 0, is captured the moment the paint fragments become wetted with solvent in the meniscus between the basin and the stage micrometer. The t = 0 image defines the initial paint fragment dimensions from which changes in area are determined. As they swell in the solvent, images of the samples are then taken at regular intervals over a time period ranging from 40 to 60 minutes for the faster-diffusing solvents like acetone, to 180 minutes or more for slower diffusers, or even for periods as long as 24 hours for aqueous liquids. Focus and magnification are not adjusted during an experiment in order to ensure internal consistency of images, and the scale of the cover slide is included in the field of the images for calibration purposes. All images of the samples immersed in solvent are captured ostensibly under constant conditions of illumination and viewing. As mentioned previously, the microscope lamp is illuminated only when necessary to capture an image of the samples in order to prevent heating the solvent and paint samples. During the course of the swelling experiment, the basin is covered to restrict evaporation and evaporative cooling of the samples. The initial volume of solvent in which the samples are immersed is generally on the order of 10 ml. No additional solvent is added to the basin during the course of the swelling experiment for reasons that are explained below. The stock of test solvent is always conditioned to the ambient room temperature to limit any thermal effects. Clearly, the absolute resolution of area measurement will be dependent on the degree of magnification employed for a given experiment. There are, however, advantages to using lower magnifications, for lower magnification provides a broader field of view through the microscope and, consequently, the possibility of including more sample fragments in a single swelling experiment. The choice of magnification is, therefore, something of a compromise of dimensional resolution and quantity of data/statistical validity. In practice, differential sample response is by far the strongest cause of variability in measured results. For the majority of experiments conducted so far, magnifications of 1.5–2.0x are generally employed, which allow 8–10 paint fragments ca. 2–5 mm2 to be measured for swelling simultaneously and with reasonable accuracy. In terms of absolute resolution, at an objective magnification of 2.0x, one pixel corresponds to an area on the order of 0.000124 mm2. Higher magnifications are, of course, perfectly possible, but these give reduced fields of view and so constrain the size or number of samples that may be viewed simultaneously. Where possible, following the approach of Eissler and Princen (1968, 1970), the small paint fragments under test are triangular in shape. However, the more brittle films often fracture into shapes other than triangular, but paint fragment shape has been found to exert little influence on the area-swelling measurements. All paint samples of a given type are substantially the same thickness. Thickness ranges of the paint films examined are The raw data, then, from a swelling experiment are a set of images of paint fragments at different times and degrees of swelling. A typical raw image is shown in figure 2a. For strong-swelling solvents, the dimensional changes that samples undergo during the course of swelling are clearly perceptible on a purely visual basis. Typically, 10–15 images are collected per experiment, with images captured more frequently in the early stages when the rate of swelling is greatest. All the sample images thus captured are image-processed as a group: the same operations are applied to all images in an experiment set. The first stage in the electronic processing is adjustment of brightness and contrast to render the paint fragments as distinct dark particles against a white background, which is done in Adobe Photoshop 5.0 (see fig. 2b). The same brightness and contrast levels are applied to all the images of immersed samples. Sharpening filters may also be applied to the digitized images if thought to improve image quality. Again, the same filter is applied to all images in a set to ensure internal consistency.
Quantitative data on the size of the individual paint fragments cannot be extracted directly using Adobe Photoshop, and to measure the changes in paint fragment dimensions the images are exported to Graftek Optilab 2.6.1. This software package has been used previously to measure changes in weave geometry parameters of artist's canvases as a function of RH (Bilson 1996). The images of paint fragments immersed in solvent are first calibrated for dimension, as a group or individually, against the stage micrometer. They are then thresholded to render the paint fragments as discrete particles against a dark background, as in figure 2c. The Optilab software has functions for detecting discrete particles and for defining their dimensions in terms of more than 20 different parameters. At the resolutions operating in the present method, sample perimeter as an indicator of swelling—which was determined microscopically in the method of Eissler and Princen (1968, 1970)— was found to involve a high degree of variability due to differential pixelation in the image capture and processing operations. Particle area has been found to be a far more reliable descriptor of paint fragment swelling. The absolute values of particle area measured at each time interval are converted to % change in area by simple calculation against the t = 0 values
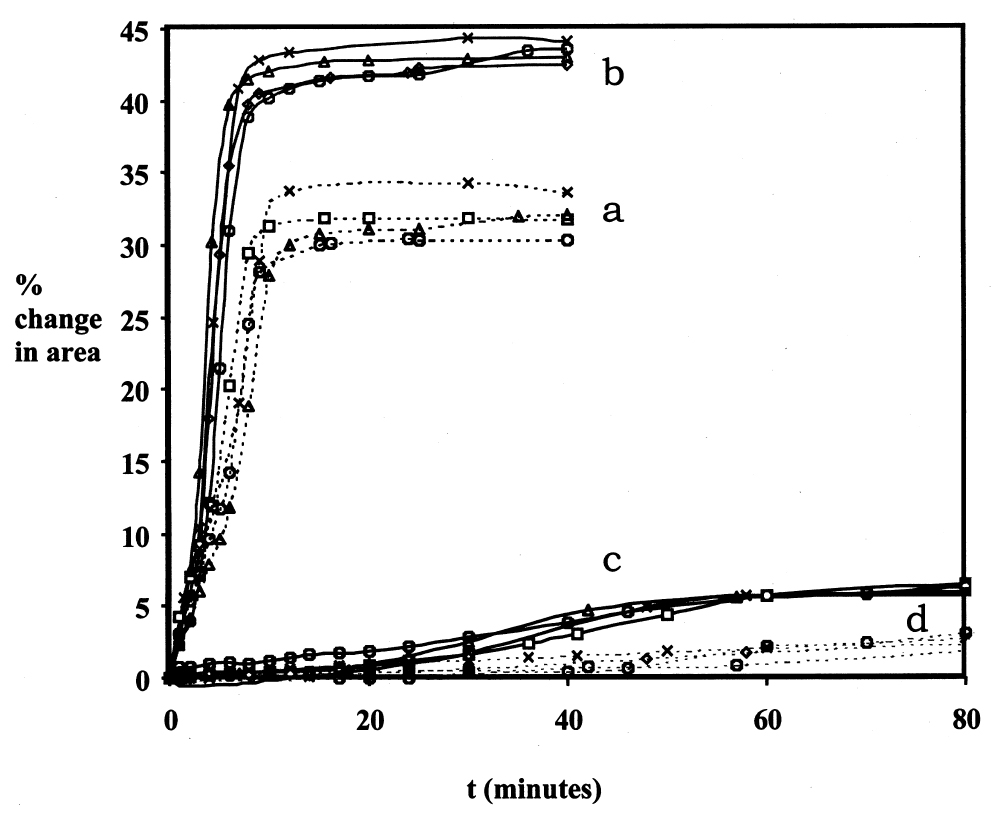
3 SOURCES OF ERRORThe technique described above measures swelling by the change in a gross physical property, particle area, due to sorption of solvent. Inevitably, a technique that is so direct and so simple in essence involves many possible sources of random and systematic error in the measurements. It is necessary here to consider the more significant issues concerning the reliability of the data, to identify the major sources of error and variability, and to discuss how these are dealt with. Three main aspects of uncertainty in the data are worthy of special consideration: temporal inconstancy of the imaging system, repeatability and accuracy, and the competing processes of leaching and swelling. 3.1 TEMPORAL INCONSTANCY OF THE IMAGING SYSTEMAn essential premise of the method is that the constant imaging conditions for all images constituting a single experiment set ensure a high degree of internal reliability in the measurement of relative paint fragment areas, both within one image frame and from one frame to the next. Put another way, within a series of images of paint particles captured over time, the dimensions of the image field of view and the boundary conditions of the particles are assumed to be constant. Any apparent change in the dimensions of a fragment image is therefore assumed to reflect a real change in actual paint fragment dimension. The absolute values of sample dimension are not, in practice, the critical parameters but rather the proportional change in dimension over time. It has been found, however, that image constancy is not perfect and that the measured area even of an inert particle can vary slightly over time. Through numerous experiments involving groups of inert particles, both immersed in liquid and unimmersed, it has been established that the inconstancy is largely systematic in character: a similar pattern and magnitude of inconstancy over time is usually observed for all particles in a group. The cause of this systematic error appears to be fluctuation in the brightness of the tungsten-halogen lamp, which leads to slight variation in boundary determination from one image to the next. The relatively low resolution of the charge coupled device (CCD) video cameras used here tends to contribute to this aspect of error due to the relatively coarse pixelation at the paint particle boundaries. It is believed that the higher resolution offered by still digital cameras will provide much greater image constancy. Future work, therefore, will use that form of image capture, rather than video, for reasons described later in the text. The temporal inconstancy is not, however, 3.2 REPEATABILITY AND ACCURACY: SAMPLE VARIABILITYVariability in measured swelling response (both rate of swelling and magnitude of maximum or equilibrium swelling) will inevitably derive from factors related to the paint fragments themselves. Size, shape, surface area, thickness, etc. will all have some influence on the specific swelling behavior of an individual paint fragment. Therefore, the source paint films for experiments of this kind must be as homogeneous as possible. An advantage of the method presented here is that several paint fragments of the same type can be tested simultaneously, giving improved statistical coverage without the need for many repeats. It is important, however, to assess the magnitude of variability within a typical sample set and the repeatability of results. The typical performance of the method in these respects is demonstrated in figure 3. The data presented in figure 3 are for two paint films (1 and 2) in the very high-swelling solvent N-methyl-2-pyrrolidone and for two paint films (16 and 17) in the low-moderate–swelling solvent butan-2-ol. For each paint-solvent combination, the mean swelling curves are shown from each of four repeat experiments. The data on maximum degree of swelling are summarized in table 2. In some instances the standard deviation of measurements of maximum % swelling within a single experiment can be relatively high in proportion with the magnitude of swelling, as in the case of the results for paint sample 16 in butan-2-ol, experiment 1. It can be seen, however, that by a relatively small number of repeat experiments the standard error can be reduced significantly1 (Miller and Miller 1993). As might be expected, the error in the measurement of maximum swelling is proportionally greater for the low-swelling cases but generally of the order of 10% of the mean value or less. This benchmark, which seems not unreasonable for a method such as this, has been used as a guide for the number of samples and repeat experiments required for particular paintsolvent combinations. 3.3 THE COMPETING PROCESSES OF LEACHING AND SWELLINGIt was demonstrated in the work of Stolow that leached paint films—that is, those from which soluble low molecular weight organic components in the paint binder have been removed by solvent extraction—usually show a different swelling response to unleached,“virgin” films (Feller et al. 1985, 61). It has been found in the present study that the phenomenon of leaching can be a significant contributor to variability in the measured swelling response of paint fragments of a given type, especially virgin films. The processes of swelling and leaching are, in some respects, in competition: increase in the dimension of a paint sample due to solvent sorption may be more or less countered by the contraction associated with
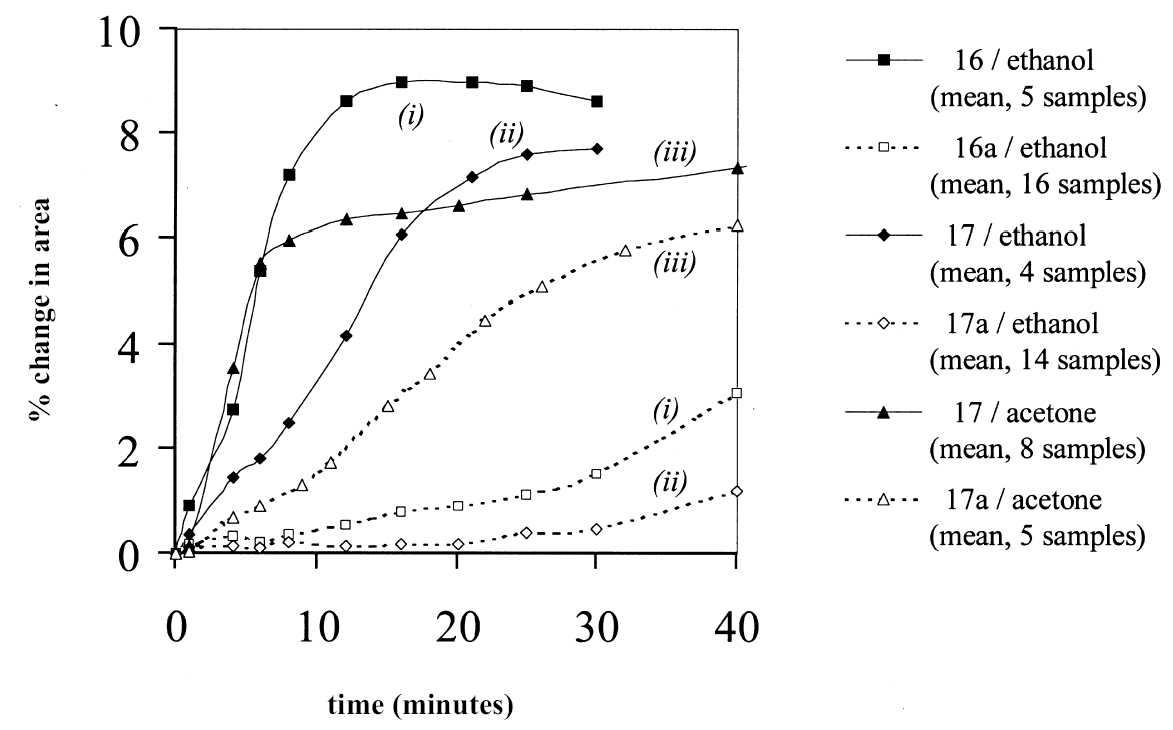
The contribution of the soluble organic phase of a paint film to the dynamic swelling response can be illustrated by comparing the swelling curves of leached and virgin paint films. Figure 4 shows the swelling behavior in ethanol of virgin samples of paint films 16 and 17 in comparison with samples from the same stock (16a and 17a) that have been extracted by immersion 10 days in acetone, followed by drying in ambient conditions for 25 days. The difference in response of the virgin and leached paint films in ethanol is really quite marked. It can be seen that, for both paint types, the preleached films swell at a much slower rate and, initially at least, to a much lesser extent than the unextracted films. Maximum degrees of swelling for the virgin paint films 16 and 17 are 9.0% and 7.7% respectively, and these values are reached quickly (in approximately 10 minutes and 25 minutes, respectively). In contrast, the preleached films have still not reached maximal/equilibrium swelling within 40 minutes, after which time the experiments are ended. The low
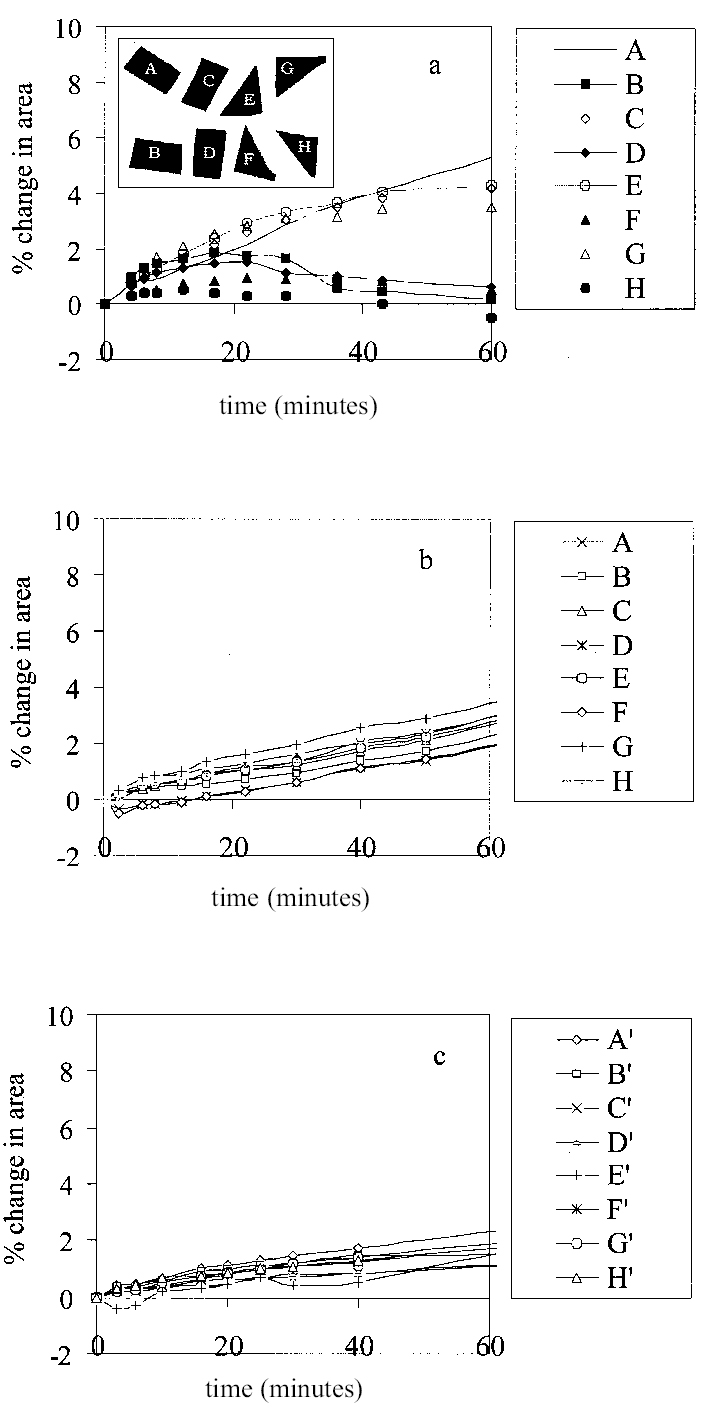
The problem leaching presents with regard to uncertainty in swelling measurements, especially on virgin paint films, is that differential rates of leaching among individual paint samples in a single experiment may contribute to appreciable variability in the measured changes in area. An experiment intended to examine this issue and the influence of sample morphology yielded results that were unexpected, but which ultimately helped to improve the experimental method. Figure 5a shows the swelling curves for eight fragments of paint film 1 (burnt umber, thermally aged) immersed simultaneously in the ether-ester solvent ethoxypropyl acetate (ethoxypropyl ethanoate). Unusually variable swelling responses had been observed in a series of experiments using this solvent, as well as other esters, and it was thought that sample shape might have been a contributing factor. As illustrated in Figure 5a, four of the paint samples were cut as rectangular
In contrast, samples B, D, F, and H initially showed a lower magnitude of swelling (< 2%), and their dynamic swelling response was characterized by a progressive decline in dimension after the maximal value had been reached, returning almost to the original area within 60 minutes—behavior that indicates leaching. This latter group of samples, which includes both triangular and rectangular shapes, all lie in the lower part of the image area, and one might look to spatial factors that could contribute to different swelling behavior of samples on one side of the “cell” compared to the other. During the course of this particular experiment, fresh solvent was delivered to the cell to compensate for solvent lost by evaporation and to dislodge bubbles that had formed around the samples. The pipette supplying the fresh solvent was located on the side of the cell closer to samples B, D, F, and H. Delivering fresh solvent to the system in this way effectively flushed the cell from one side, preferentially reducing the concentration of soluble compounds in the vicinity of samples B, D, F, and H, thereby promoting further desorption. It seems likely, therefore, that for samples B, D, F, and H leaching was accelerated and enhanced. As demonstrated earlier, the presence of soluble compounds in the paint is associated with greater degrees of swelling: therefore, preferential leaching of B, D, F, and H led to lower apparent changes in dimension. To examine if leaching had contributed to the variability in the swelling of these samples, they were left in the solvent ethoxypropyl acetate for 24 hours, after which time it was assumed they would be substantially extracted. They were then oven-dried (35�C, 24 hours), and the swelling experiment in ethoxypropyl acetate was repeated. The swelling curves for the eight samples in the repeat experiment are shown in Figure 5b. As seen previously, the swelling of the leached samples proceeds at a slower rate than does the swelling of virgin paints: the samples were still not at equilibrium after 70 minutes, when the experiment was terminated. All of the eight samples now swelled at essentially the same rate, as indicated by the gradient of the curves, and although there is some spread in the magnitude of swelling, it is comparable for all eight samples. At t = 70 minutes the mean value of % change in area is 3.13%, s.d. 0.56%, which is a more acceptable level of precision. Comparable effects were also observed when paint samples of type 1 were preextracted by immersion in acetone rather than ethoxypropyl acetate. Figure 5c shows the swelling curves, in ethoxypropyl acetate, of eight different samples (A'–H') of thermally aged burnt umber paint film 1 preleached by immersion 24 hours in acetone. Although the swelling curves are somewhat less linear than the previous case, the overall behavior is similar. The mean value of maximal swelling and the standard deviation are comparable in order of magnitude with the previous case. A key conclusion from these observations is that leaching may, with some paint-solvent combinations, be a significant contributor to experimental variability using the present method. Since addition of fresh solvent during the course of an experiment is shown to magnify the problem, this operation is avoided. In a preliminary report on this work (Phenix 1998), the low apparent swelling power of a particular, relatively polar solvent (designated solvent X) was noted. This solvent was ethoxypropyl acetate. The low values of swelling observed initially, it is now understood, were due in part to a substantial amount of leaching occurring during the swelling experiment. Ethoxypropyl acetate, as will be demonstrated in a subsequent article, is low-moderate in its swelling power on the paint films studied here (Phenix 2002, Part 2). Even so, with some of the paint films examined during this study the variability in measured values of maximum/equilibrium swelling can be so high, even with numerous repeat experiments, that the data are rather unreliable as an indicator of the magnitude of paint-solvent interaction. This conclusion applies, especially, to some of the films with very high medium content and high proportions of soluble
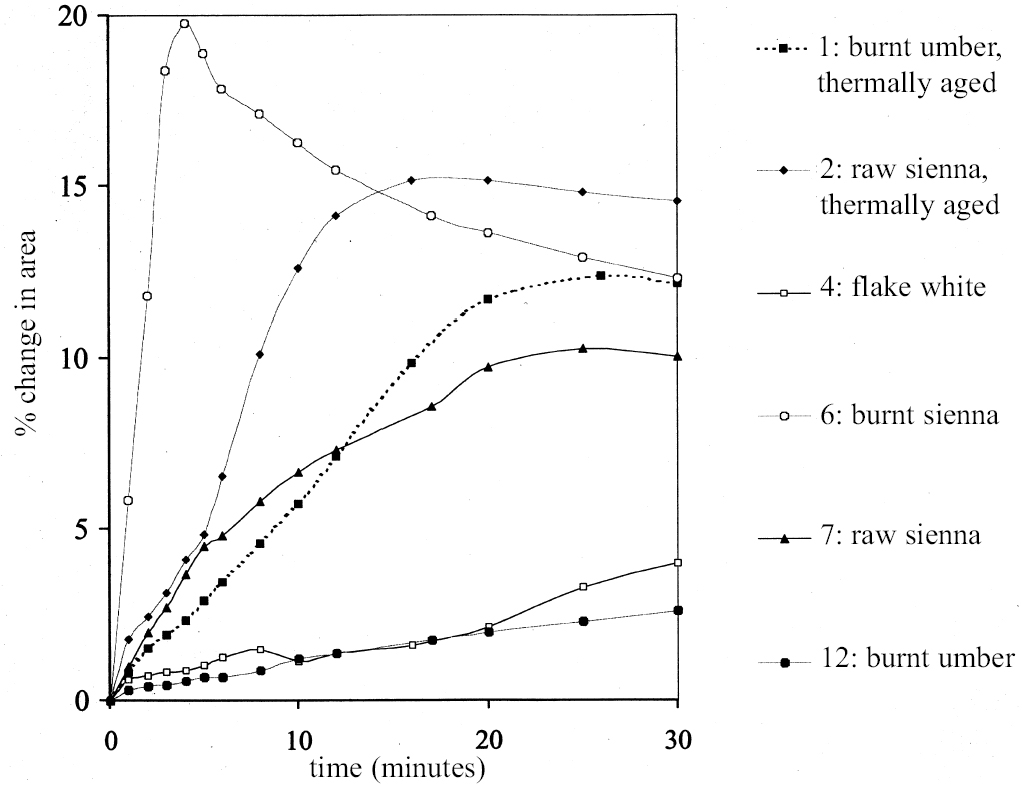
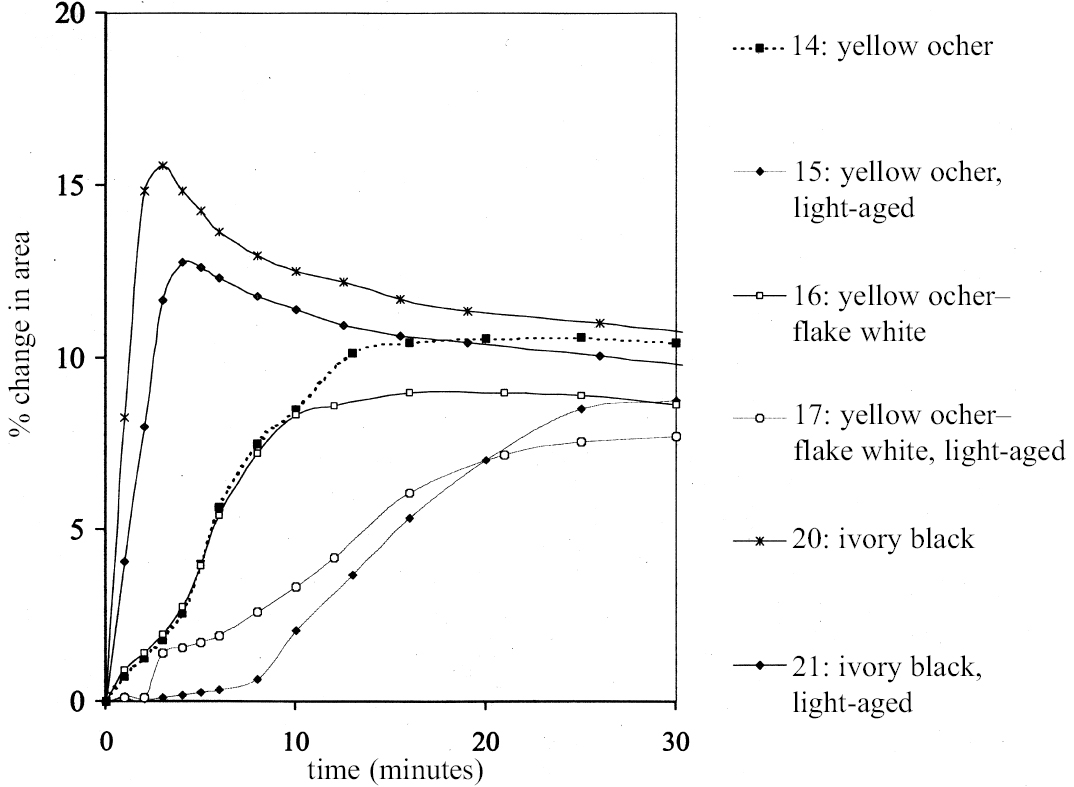
4 SWELLING OF PAINT FILMS OF DIFFERENT TYPESFigures 6 and 7 show the swelling curves, up to t = 30 minutes, obtained for samples from reference paint films of different types when immersed in ethanol. These samples come from three separate sets prepared from conventional artist's paints based on drying oils. Included are paints that are naturally dark-aged and paints subjected to some form of accelerated aging, either by exposure to high-intensity light or to heat. They are all essentially proprietary artist's oil paints, or mixtures thereof, cast as films of uniform thickness on polyester film supports. The paints vary significantly in their constitution and consistency. Small fragments of paint can be readily removed from the polyester film as required for each experiment. The experiments reported here refer to a selection of 12 different paint films from the above sets, each defined by number (1, 2 etc.). The details of each individual paint film type are given in table 1. It can be seen immediately that paint films vary appreciably in both the rate and the extent of swelling in a given solvent. Some of the paint films (e.g., 4: flake white, and 12: burnt umber) still had not reached equilibrium after 30 minutes, and both these paint films showed only low degrees of swelling in ethanol, < 5% in area. In contrast, some of the other paint films (e.g.,6: burnt sienna, 20: ivory black unexposed to light, and 21: ivory black exposed to light) showed very rapid swelling, maximum swelling being reached within 5–6 minutes. Thereafter these paint films contract from the maximum degree of swelling to an equilibrium value. Both these factors suggest paint films that are lightly cross-linked, with high medium content and an abundant soluble phase that is susceptible to extraction by the solvent. The other paint films swell progressively, but at a slower rate, to the equilibrium value without passing through such a distinct contraction stage. It is interesting to note that most of the paints composed of iron oxide earths, especially the siennas, show moderate-to-high degrees of swelling. The very high solubility of these sienna paints has been demonstrated previously (Erhardt and Tsang 1990, table 2, 96; Hedley et al. 1990). The difference between the two sets of samples of Winsor & Newton raw sienna (2 and 7) is explained partly by their different aging histories and partly by their thicknesses. The same also applies to the two burnt umber paint films (1 and 12). It is useful to note at this point the issue of paint film aging insofar as this influences swelling behavior. It can be seen from figure 4 that the paints that have been aged by exposure to light show lower degrees of equilibrium swelling and a slower rate of swelling compared to their unexposed counterparts (compare, for example, the curves for 14 vs. 15, 16 vs. 17, and 20 vs. 21). This finding applies both for the virgin and for the preleached films. This pattern has been found with virtually all solvents and is presumably due to several factors: increased paint density in the lightexposed samples, lower organic content, and, possibly, higher cross-link density. It is possible, also, that the presence of a mobile, soluble phase within the organic binder actively promotes solvent penetration in the paint binder network. Paint films 16 and 17, which of the group reported here are intermediate in character, have been the subjects of more extensive investigations reported in Phenix 2002. Given the variability in the swelling behavior of paint films of different kinds demonstrated above, it follows that comparison of swelling response between one paint film and another presents certain difficulties unless the samples share a common origin. However, the variation in swelling response of a single paint film type with solvents of different kinds gives considerable insight into the chemical nature of the paint and provides the basis of a framework to enable the conservator to assess and control risk in the process of cleaning.
5 SUMMARYIt has been demonstrated that the method proposed here, although very simple in essence, can provide a valid means of comparing the swelling response of a given paint film in a range of organic solvents. Uncertainty in the measured swelling response derives from a number of factors related both to the measurement process and to the variability in behavior of individual paint fragments. The latter element is by far the greatest contributor to experimental uncertainty. It has been shown that by careful selection of sample material, by control of the experimental process, and by using multiple samples of the same paint type, uncertainty can be reduced to acceptable levels so that the method is able to discriminate the different swelling powers of solvents, at least on young-mature paint films. ACKNOWLEDGEMENTSThis work was commenced during a period of secondment to the MOLART (Molecular Aspects of Aging in Painted Works of Art) project, a Prioriteit research program funded by the Dutch Organization for Scientific Research (NWO) and organized by the Foundation for Fundamental Research on Matter, Institute for Atomic and Molecular Physics (FOM-AMOLF), Amsterdam. I gratefully acknowledge the support of the Courtauld Institute of Art for facilitating this period of secondment. The work could not have been undertaken without the equipment acquired with the aid of a grant from the Research Committee of the Courtauld Institute of Art. Special thanks must also go to Tom Bilson of the Courtauld Institute of Art for assistance with the digital imaging and analysis and to Ken Sutherland for his gas chromatography–mass spectroscopy work on the samples that form the subject of this article. I am indebted to Steen Sauerberg, University of Copenhagen, without whom this work would not have seen the light of day. NOTES1. The measurements from separate experiments can be considered as a sample from an infinite population of possible measurements; and, likewise, the means of any series of measurements are a sample from the possible means of samples of measurements from the whole population. The distribution of the sample means is called the sampling distribution of the mean: its mean is the same as the mean of the original population. The standard error of the mean (SEM) is equivalent to the standard deviation of the sampling distribution of the mean (Miller and Miller 1993). REFERENCESBarr-Howell, B. D., and N. A.Peppas1985. Dynamic and equilibrium swelling of DVB crosslinked polystyrene particles. Journal of Applied Polymer Science30:4583–89. Bilson, T.1996. Canvas shrinkage: A preliminary investigation into the response of a woven structure. ICOM Committee for Conservation preprints, ed. J.Bridgland, 11th Triennial Meeting, Edinburgh. London: James and James. 1:245–52. Bristow, G. M., and W. F.Watson1958. Cohesive energy densities of polymers. Part 1, Cohesive energy densities of rubbers by swelling measurements. Transactions of the Faraday Society54:1731–41. Browne, F. L1956. Swelling of oil paints in water. Part 8, Swelling of linseed oil paints in water and organic liquids. Forest Products Journal6:312–18. Brunt, N. A1964. Blistering of paint layers as an effect of swelling by water. Journal of the Oil and Colour Chemists' Association47:31–42. Byun, J., Y. M.Lee, and C. S.Cho1996. Swelling of thermosensitive interpenetrating polymer networks composed of polyvinylalcohol and polyacrylic acid. Journal of Applied Polymer Science61(4):697–702. Chen, W. L., K. R.Shull, T.Papatheodorou, D. A.Styrkas, and J. L.Keddie1999. Equilibrium swelling of hydrophilic polyacrylates in humid environments. Macromolecules32:136–44. Down, J. L1999. Swelling as an indicator of removability. In Reversibility: Does it exist?ed. A.Oddy and S.Carroll. London: British Museum. 111–27.
Eissler, R. L., and L. H.Princen1970. Swelling of linseed oil films in acid and alkaline environments. Journal of Paint Technology42(542):155–58. Erhardt, D., and J-S.Tsang1990. The extractable components of oil paint films. In Cleaning, retouching, coatings, ed. J. S.Mills and P.Smith. London: International Institute for Conservation. 93–97. Errede, L. A1992. Polymer swelling. Part 11, A molecular interpretation of association in polystyrene-liquid systems. Polymer33(19):2168–76. Faraday Society1946. Swelling and shrinking: A general discussion.Edinburgh: Gurney and Jackson. (Reprint of the Transactions of the Faraday Society 42B [1946].) Feller, R. L., N.Stolow, and E. H.Jones1985. On picture varnishes and their solvents. Rev. and enlarged ed. Washington, D.C.: National Gallery of Art. Gee, G1942. The interaction between rubber and liquids. Part 3, The swelling of vulcanised rubber in various liquids. Transactions of the Faraday Society3:418–22. Green, A., and G. I. P.Levenson1972. A practical swellmeter. Journal of Photographic Science20:205–10. Hedley, G., M.Odlyha, A.Burnstock, C.Husband, and J.Tillinghast1990. A study of the mechanical and surface properties of oil paint films treated with organic solvents and water. In Cleaning, retouching, coatings, ed. J. S.Mills and P.Smith. London: International Institute for Conservation. 98–105. Huglin, M. B., and D. J.Pass1968. Cohesive energy density of polytetrahydrofuran. Journal of Applied Polymer Science12:473–85. Johnsen, J. S1996. Accelerated aging: Changes in swelling and melting point of photographic gelatin. ICOM Committee for Conservation preprints, ed. J.Bridgland, 11th Triennial Meeting, Edinburgh. London: James and James. 2:586–90. Lewis, E. J., and A. K.Soper1950. Note on an apparatus for measuring the vertical swelling of colloid layers. Journal of Scientific Instruments31(27):242–43. Long, J. S., S.Thames, and O.Smith1967. Surface forces and dimensions. Journal of Paint Technology39(507):169–81. Mangaraj, D1963. Cohesive energy densities of high polymers. Part 1, Determination of cohesive energy density from swelling measurements. Makromolekulare Chemie65:29–38. Michalski, S1990. A physical model of the cleaning of oil paint. In Cleaning, retouching, coatings, ed. J. S.Mills and P.Smith. London: International Institute for Conservation. 85–92. MillerJ. C., and J. N.Miller1993. Statistics for analytical chemistry.3d ed.London: Ellis Horwood. Morehead, F. F1952. A method for studying the effect of humidity on the cross-sectional swelling of some common fibers. Textile Research Journal22:535–39. Nguyen, T.-P., B.Lavedrine, and F.Flieder1999. Effets de la pollution atmosph�rique sur la d�gradation de la g�latine photographique. ICOM Committee for Conservation preprints,ed. J.Bridgland, 12th Triennial Meeting, Lyons. London: James and James. 2:567–71. Parbhoo, B., S.Izrael, J. M.Salamanca, and J. L.Keddie. 2000. Use of ellipsometry and gravimetry to develop standards for measuring silicone coat weight and thickness with x-ray fluorescence spectroscopy. Surface and Interface Analysis29:341–45. Parker, L. V., and T. A.Ranney1995. Additional studies on the softening of rigid PVC by aqueous solutions of organic solvents. U.S. Army Corps of Engineers, Cold Regions Research and Engineering Laboratory. Special report 95-8.
Parker, L. V., and T. A.Ranney1996. Further studies on the softening of rigid PVC by aqueous solutions of organic solvents. U.S. Army Corps of Engineers. Cold Regions Peppas, N. A., and E. W.Merrill1976. Determination of interaction parameter χ1 for poly (vinyl alcohol) and water in gels crosslinked from solutions. Journal of Polymer Science, Polymer Chemistry Edition14:459–64. Phenix, A1998. Solvent-induced swelling of paint films: Preliminary results. Western Association for Art Conservation Newsletter20(3):15–20. Phenix, A.2002. The swelling of artists' paints in organic solvents. Part 2, Comparative swelling powers of selected organic solvents and solvent mixtures. Journal of the American Institute for Conservation41:61–90. Rinse, J., and W. H. G.Wiebols1937. Swelling of drying oil films in water. Industrial and Engineering Chemistry29(10):1149–54. Salomon, G., and G. J.van Amerongen1947. Influence of structure on polymer-liquid interaction. Part 1, Relative and absolute values of swelling equilibria. Journal of Polymer Science2:355–70. Scott, R. L., and M.Magat1949. Thermodynamics of high polymer solutions. Part 3, Swelling of cross-linked rubber. Journal of Polymer Science4:555–71. Stolow, N1954. A modified apparatus for measuring the swelling of polymer films in solvents. Journal of Scientific Instruments31:416–20. Stolow, N1955. Some investigations of the action of solvents on drying oil films. Ph.D. diss., Courtauld Institute of Art, University of London. Stolow, N1963. Application of science to cleaning methods: Solvent action studies on pigmented and unpigmented linseed oil films. In Recent advances in conservation, ed. G.Thomson. London: Butterworths. 84–88. Tan, N. C. B., W. L.Wu, W. E.Wallace, and G. T.Davis1998. Journal of Polymer Science, Part B: Polymer Physics36:155. Thiessen, P. A1925. Methoden zur quantitativen Bestimmung von Quellungsgr��en. Kolloid Zeitschrift37:406–11. Zellers, E. T., D. H.Anna, R.Sulewski, and X.Wei1996a. Critical analysis of the graphical determination of Hansen's solubility parameters for lightly crosslinked polymers. Journal of Applied Polymer Science62:2069–80. Zellers, E. T., D. H.Anna, R.Sulewski, and X.Wei1996b. Improved methods for the determination of Hansen's solubility parameters and estimation of solvent uptake for lightly crosslinked polymers. Journal of Applied Polymer Science62:2081–96. AUTHOR INFORMATIONALAN PHENIX was educated at Leeds University, United Kingdom, receiving a B.Sc. Honors in chemistry and color chemistry combined in 1979. He studied conservation of easel paintings for three years at the Courtauld Institute of Art, University of London, and was awarded the institute's postgraduate diploma in 1984. After an internship at Tate Gallery, London, he worked as a paintings conservator in Australia, returning to Britain in 1989 to take up a teaching and research position at the Hamilton Kerr Institute, University of Cambridge. He was a lecturer in the Department of Conservation and Technology at the Courtauld Institute of Art from 1991 to 2000, during which time he spent 15 months on secondment to the MOLART (Molecular Aspects of Aging in Painted Works of Art) project managed by the Foundation for Fundamental Research on Matter, Institute for Atomic and Molecular Physics (FOMAMOLF), Amsterdam. During 2001 he was research fellow at the Royal College of Art, London, and he has recently been appointed associate professor at the Institute for Archaeology, Art History and Conservation, at the University of Oslo. He is presently coordinator of the ICOM-CC Working Group Paintings 1: Conservation and Restoration of Paintings. Address: Department of Conservation Studies, Institute for Archaeology, Art History and Conservation (IAKK), University of Oslo, Frederiks gt. 3, 0164 Oslo, Norway.
 Section Index Section Index |