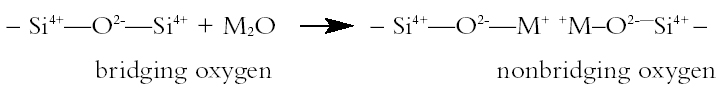THE CONSERVATION OF WET MEDIEVAL WINDOW GLASS: A TEST USING AN ETHANOL AND ACETONE MIXED SOLVENT SYSTEMD. R. GRIFFITHS, & A. M. FEUERBACH
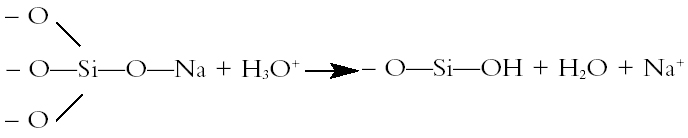
1 INTRODUCTIONThe conservation procedure proposed in this article was developed in response to the need to conserve an assemblage of excavated, wet, painted medieval window glass fragments. They appeared on art-historical grounds to date from a variety of periods. The treatment had to remove the water in which the fragments had been stored since excavation, consolidate the deteriorated pieces, and leave them in a fit state for long-term storage. While achieving these aims, the treatment also had to be inexpensive, require no specialized equipment, be relatively quick to perform, and involve a minimum of health and safety risks. The proposed treatment removes the water by replacing it with ethanol and consolidates the fragments with Paraloid B-72 (an acrylic polymer) dissolved in a mixed ethanol and acetone solvent system. Examination of the stored samples five years after this treatment indicates that the method appears successful. It may thus be worthy of further consideration and testing by others who need to conserve glass in a similar condition. To explain some of the rationale behind the treatment proposed, the article includes a brief description of the mechanisms by which archaeological glass is attacked, the compositional and environmental factors influencing the rate of deterioration, and some of the features that these deterioration processes create. Although some of this information
|