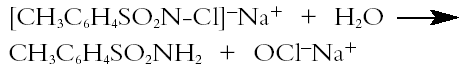SHORT COMMUNICATION: MICRO-RAMAN IDENTIFICATION OF BLOOM FORMED ON A HISTORICAL PRINT ARTIFACTVINCENT OTIENO-ALEGO, JENNIFER HODGEMAN, & DUDLEY C. CREAGH
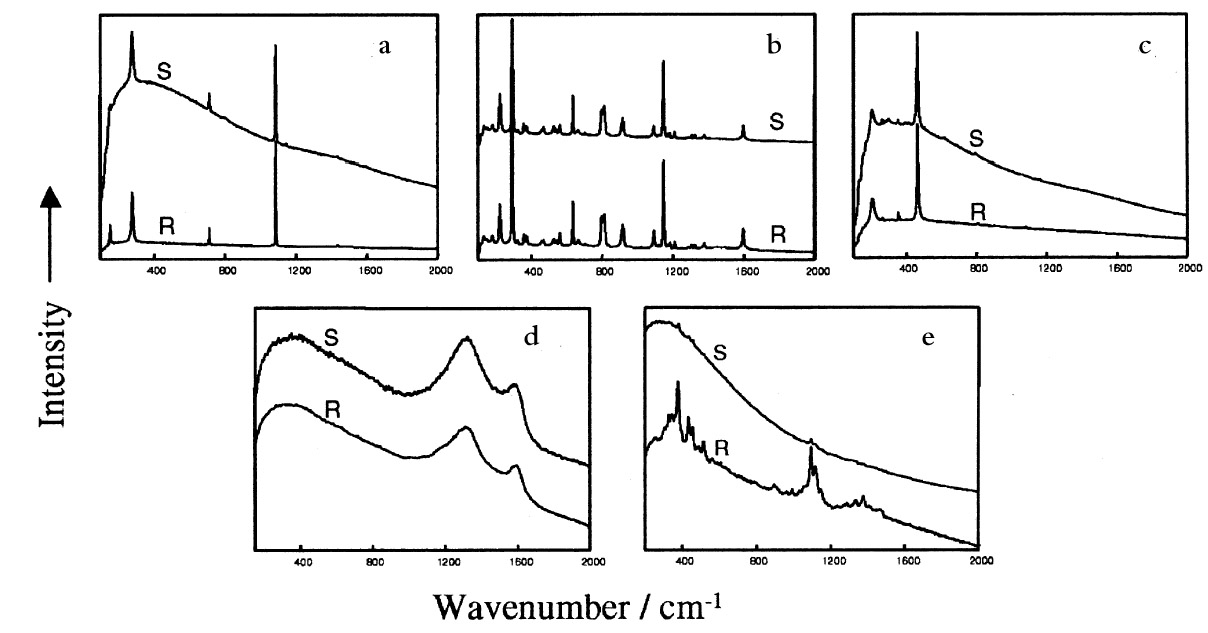
3 RESULTS AND DISCUSSIONFigure 2 shows a typical white light micrograph of the powdery deposit examined under a light microscope using a 10x microscope objective. This examination revealed three distinct types of crystalline material. The deposit included relatively large transparent crystals (see example labeled A in fig. 2) dispersed amid the bulk of much smaller, more rounded white crystals (example labeled B, fig. 2) and a few orange-colored crystals (example labeled C). Some black particles (labeled D) were also observed, as well as white fiberlike materials (labeled E) whose lengths ranged between 50 and 200 �m. These particles hereafter will be referred to simply as solids A, B, C, D, and E, respectively. Raman microanalysis of all the solid particles enclosed in the predefined area of 1200 x 1200 �m2 was undertaken by focusing the laser onto the individual particles via the microscope objective. Identification
The occurrence of paratoluenesulfonamide may be linked to the use of chloramine-T (the sodium derivative of N-chloro-p-toluenesulfonamide: ([CH3C6H4SO2N(Cl)Na]), which was a popular paper bleaching reagent in the 1960s and 1970s (Lienardy and Van Damme 1990).When dissolved in ethanolic solution, chloramine-T slowly hydrolyzes, releasing hypochlorite ions (Corrigan 1997) according to the following equation:
Thus, the bleaching action of chloramine-T is comparable to that of sodium hypochlorite, except that in this case the bleaching process is much slower and therefore more controllable. The Cl-free toluenesulfonamide identified in this study is a product of this cleavage reaction. Chloramine-T bleaching was considered to be simple, efficient, safe, and less harmful to the paper (Hey 1977). Paper treated with chloramine-T required only a simple rinse because traces of the chemical were presumed to have a bactericidal action long after the treatment (Lienardy and Van Damme 1990). The occurrence of its residues on this print artifact is thus not surprising. The idea that chloramine-T is safe has since been amended. Chloramine compounds are unstable, and the residual The black carbon particles most likely came from the print ink, while the cellulose fibers broke loose from the aging paper. The source of the few quartz crystals identified in the white solid deposit is probably trapped dust/sand particles from the environment. |