DIGITAL VIDEO MICROSCOPY: A PRACTICAL VISUAL ANALYSIS TECHNIQUE FOR THE CONSERVATORTED STANLEY
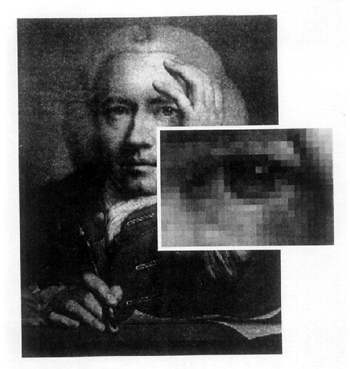
ABSTRACT—ABSTRACT—Video microscopy has been an important application in science, medicine, and industry. It has also been used in many conservation laboratories as part of the daily routine of examining and treating objects. These microscopy systems are normally composed of a cooled charged coupled device (CCD) video camera and a high-resolution color monitor used together with a microscope. Further capabilities are gained by digitizing such systems with the addition of a video capture card and computer software. This enhancement makes it possible to approximate the side-by-side visual comparison capabilities of very expensive comparison microscopes used in the forensic sciences. Such capabilities can be a valuable tool in today's conservation laboratory. It is possible to use side-by-side visual comparison studies in the course of identifying materials, examining various interactions between materials during pretreatment spot-testing, or monitoring the actual treatment phase. This article provides a brief overview of digital video microscopy and examines some of its applications that may be suitable for routine conservation use. TITRE—Vid�o microscopie digitale: une technique d�examen visuel pratique pour le restaurateur. R�SUM�—La vid�o microscopie est un outil important en science, en m�decine et dans l�industrie. Elle est utilis�e quotidiennement depuis un certain temps dans plusieurs laboratoires de restauration lors de l�examen et du traitement des objets. Ces syst�mes de microscopie sont normalement compos�s d'une cam�ra vid�o avec dispositif CCD et d'un moniteur en couleur RBV utilis�s en conjonction avec un microscope. Des capacit�s suppl�mentaires sont gagn�es en num�risant de tels syst�mes avec l'ajout d�une carte de saisie vid�o et d'un logiciel d'ordinateur. Ce perfectionnement permet d��muler les capacit�s visuelles de comparaison des microscopes comparatifs on�reux utilis�s dans les sciences l�gales. De telles capacit�s peuvent �tre un outil pr�cieux dans le laboratoire de restauration d'aujourd'hui. Il est possible d'utiliser des �tudes visuelles comparatives au cours de l�identification des mat�riaux, d�examiner diverses interactions entre les mat�riaux lors des tests ponctuels qui pr�c�dent le traitement, ou de surveiller la phase de traitement. Cet article fournit une br�ve vue d'ensemble de la vid�o microscopie digitale et examine certaines des applications qui pourraient devenir courantes en restauration. TITULO—Microscop�a digital de v�deo: una t�cnica pr�ctica de an�lisis visual para el conservador. RESUMEN—La microscop�a de v�deo ha tenido aplicaciones importantes en la ciencia, medicina, e industria. Desde hace un tiempo tambi�n ha sido usada en muchos laboratorios de conservaci�n como parte de la rutina diaria de ex�men y tratamiento de objetos. Generalmente, estos sistemas microsc�picos est�n compuestos por una c�mara con dispositivo enfriado acoplado de carga (CCD) y un monitor RGB, usados conjuntamente con un microscopio. Se puede obtener una capacidad m�s amplia al digitizar estos sistemas a�adiendo una tarjeta de captura de v�deo y un software de computaci�n. Este realce hace posible aproximarse a la capacidad de comparaci�n visual de dos im�genes simult�neas como la de los microscopios costosos usados en las ciencias forenses. Tales capacidades pueden ser una herramienta valiosa en el laboratorio de conservaci�n de hoy d�a. Por medio de ellas es posible realizar estudios de comparaci�n visual simult�nea para identificar materiales, examinar las distintas interacciones entre los materiales durante pruebas puntuales o al hacer el seguimiento durante la etapa del tratamiento mismo. Este art�culo presenta una revisi�n breve de la microscop�a digital de v�deo, y examina algunas aplicaciones que puedan ser apropiadas para su uso rutinario en conservaci�n. 1 1. INTRODUCTIONA conservator often uses microscopy to perform close visual examinations as a part of his or her daily routine. Such observations help to identify materials or choose an appropriate conservation treatment for an object. Microscopy may also help to form a long-term storage plan for more sensitive and fragile objects. Many conservation laboratories are complementing their microscope systems with cooled charged coupled device (CCD) video cameras and high-resolution color monitors. The addition of these cameras and monitors allows more than one person to view the display in excellent detail, which is very helpful in consultation or training scenarios. The systems have also been found to be a very valuable aid in treating objects (Biggs 1994). They have been extensively used in industrial, medical, and scientific research in all fields, including conservation. The capabilities of video microscopy systems can be further enhanced by incorporating a computer and a computer monitor. To link a video camera with a computer, one needs a computer peripheral such as a video capture device to digitize images. The system is invaluable when performing critical side-by-side visual comparison studies of specimens. It is equally valuable when carrying out such comparison studies as those involving the reaction of materials. This article will discuss the value of digital video microscopy in a conservation laboratory setting. 2 2. DIGITAL VIDEO MICROSCOPYMicroscopic side-by-side comparison study is a standard analytical method that has been used in forensic science for a long time. The system allows one to make critical visual comparisons of two specimens simultaneously in real-time with the same optical magnification. The method uses a comparison microscope, which is really two compound light microscopes connected by an optical bridge. An observation tube with eyepieces is in the center of the optical bridge, creating a split view of two simultaneously observed specimens. In forensic science it is critical to look for both points of identity as well as differences when performing side-by-side comparisons (Robertson 1992). The same approach is necessary when performing visual comparisons of materials in conservation. However, comparison microscopes are very expensive, so very few conservation laboratories have been able to afford them. But, at a fraction of the cost of a comparison microscope, a video capture card may be used with an existing video microscope system to quickly and inexpensively achieve side-by-side comparison capabilities close to those of a comparison microscope. The system can display on the computer monitor one or more captured images generated from the computer's data file and a real-time image from the microscope. Thus, several people may view the computer monitor at once instead of having to take turns looking into the microscope, then having to turn to look at a sample displayed elsewhere. The system is also excellent for teaching students or interns and for consulting with curatorial staff members. Imaging software such as Adobe Photoshop can enhance the quality of digital images well enough to make comparison sampling very worthwhile. However, it should be mentioned that even with a high-resolution monitor and superior software, the image quality will not be quite as sharp as looking directly into the microscope eyepieces. 2.1 2.1 VIDEO CAPTURE CARDThe key component in the system is the video capture card, an expansion card that plugs into the peripheral component interconnect (PCI) slot of the computer. It converts the analog output signals of the video camera into digital input signals for the computer to process and display. Analog technology can be described as capturing real images that are made of intensity values and positions that are continuous functions in space and time (Luther 1991). A digital image is obtained from the analog signal by a series of discrete and complex processes, which include sampling and quantization (Kabir 1996). The image is a matrix of tiny elements or squares called pixels (fig. 1). Each pixel represents a value that is a function of the information surrounding the corresponding point in the image (Poynton 1996).
A Y/C or S-video cord connects a video camera attached to the microscope via a camera mount to the video capture card by a line input on the card. The cord helps to provide high resolution by keeping separate the luminance signal (contrast) from the chrominance signal (color). The process uses more horizontal scanning lines than in standard video, which results in higher resolution. The video capture card software has controls for adjusting such features as brightness, color, sharpness, and contrast. The software is used in tandem with the latest version of Apple's QuickTime, a software architecture for creating and viewing digital media for Apple and Windows platforms (Greenberg and Greenberg 1995). The more sophisticated cards have additional video-editing software packages that include applications such as Adobe Premiere, and they cost more. Video capture cards record real-time movies running up to 30 frames per second. Also, a single frame can be frozen or captured in the process. Images can be recorded at various resolutions. Resolution indicates the number of horizontal pixels by the number of vertical pixels, for example, 640 � 480. Images are displayed using standard signal formats such as those of the National Television Standards Committee (NTSC) and Phase Alternate Line (PAL), which is an international standard in Europe and other places. 3 3. VIDEO MICROSCOPY APPLICATIONS FOR ROUTINE CONSERVATIONA conservator can take advantage of a number of practical and valuable everyday uses for video microscopy in his or her laboratory. The application of digital video microscopy is not necessarily limited to a few conservation specialty groups but can be used whenever side-by-side visual comparison studies are needed. The following are several practical instances in which I have found digital video microscopy to be an important and valuable tool. 3.1 3.1 SPOT TESTING SOLUBLE PIGMENTSSpot testing pigments is a routine examination activity that is performed by a paper conservator. Spot testing is conducted to determine a pigment's likely reaction to various reagents or liquids before an actual treatment is carried out. Once testing begins, it is always difficult to remember how a very small area, a few millimeters in diameter, truly appeared before testing. Therefore, trying to determine exactly how the pigment is being affected by the application of a reagent is sometimes problematic. The conservator has a powerful tool for this type of situation by using the side-by-side visual comparison capabilities of a digital video microscopy system. For example, a pen and ink wash by Felix Octavius Carr Darley (1822–1888) was brought to my attention by the Graphic Arts Collection curator (fig. 2). Darley worked as an illustrator in Philadelphia and New York City during the 1840s and 1850s (Groce and Wallace 1957). The drawing suffered from a nasty mat burn that obscured the drawing. An aqueous treatment was contemplated for the drawing to reduce the mat burn. The ink wash and paper support had to be spot tested before beginning any treatment to minimize the risk of ink loss and also to predict what sort of aqueous solution would be effective in reducing the staining.
The initial test was to determine a safe and effective aqueous solution for washing the object. A very tiny and obscure area of the drawing was selected for testing. A still image of the area was captured and saved on the computer before testing began. I then brought up a real-time frame image of the area to be tested beside the still-frame image on the computer monitor. Both images were 320 � 240 recorded resolutions. As I tested the area with various solutions of water and absolute ethanol, I could observe the effect of the solutions and compare it to the image of the same untested area on the computer monitor. I could also periodically print out images of the area, tested and untested, as the examination progressed to make visual comparisons of the testing (fig. 3). The images were made part of the treatment documentation in hard copy and computerized form. The same type of testing continued for finding an aqueous solution for stain reduction. Exhaustive testing found that (1:4) absolute ethanol:distilled water solutions were suitable for washing. Further, the same proportions of absolute ethanol: 1% and 2% buffered hydrogen peroxide (for immersion and local applications, respectively) proved to be safe and effective for stain reduction using the same comparison method as before.
3.2 3.2 IDENTIFICATION OF PHOTOGRAPHSDifferent types of photographic processes have developed since William Henry Fox Talbot's invention in 1839. Some of these processes can be identified through visual examination of the photograph's surface characteristics. A trained eye can pick up the subtle distinctions between one process and another, but differentiating closely related photographic processes is made easier by the use of side-by-side comparison techniques. I received several 19th- and early-20th-century photographic prints for conservation from the Princeton University Archives (Seeley G. Mudd Manuscript Library). Two photographs dated 1868 (Princeton class of 1868) and 1890 (Princeton class of 1890) were of particular interest when their surface characteristics were examined under a stereo microscope (fig. 4). At first I thought the 1868 print may have been a salt print, but, on closer inspection, I found an extremely light coating of albumen. The 1890 photograph also appeared to be an albumen print, but with a much thicker coat. The fibers of the paper appeared distinctly through the albumen layer, and there was no apparent baryta layer, which is indicative of a gelatin print (Reilly 1986). I generated magnified images of the respective surfaces on my computer and displayed them side-by-side (fig. 5). It appeared that the 1868 photograph was most likely an albumen photograph that perhaps had not been commercially prepared. The print had a very distinct textured surface because of an extremely thin coating of albumen over the paper fibers. Both prints were further examined and conserved following their identification.1
3.3 3.3 FIBER AND PIGMENT ANALYSISDigital video microscopy is a valuable aid in the examination of paper and other types of cellulosic fibers. The morphology of a fiber can be carefully observed using a polarized light microscope. Comparative observations between known and unknown specimens make the task of identification much easier to perform. A fiber sample is placed between a glass slide and cover, and a refractive index indicator medium is added (McCrone et al. 1995). Through polarized light microscopy, specific morphological characteristics of the unknown specimen can be compared to known samples that may lead to the specimen's identification. Examination of the unknown specimen proceeds by analyzing it for such characteristics as size, signs of elongation, extinction, vessel walls, refractive index, etc. There are several very useful sources of reference materials covering the morphological features of fibers, such as C�t�'s Papermaking Fibers: A Photomicrographic Atlas (1980). Using digital video microscopy, a still image of the unknown specimen is obtained and saved in the computer. The unknown specimen on the microscope stage is replaced with a known specimen that approximates or matches the morphological characteristics of the specimen. The known specimen can be compared in a real-time frame on the monitor side-by-side against the saved image of the unknown specimen. Through systematic comparative study, the unknown specimen can be identified (fig. 6). Fiber samples for carrying out comparative analysis studies can be obtained from several sources, such as the Institute of Paper Science and Technology and McCrone Accessories and Components, which also sells Cargille pigment sample kits.
Comparative pigment analysis studies can also be made in conjunction with performing standard examination protocols. Unknown-pigment specimens are compared side-by-side to known samples. Various reference sources for pigment characteristics may be consulted to help in the identification process. Walter McCrone (1982) has published an excellent reference, which has analytical data on a great number of historic artists' pigments. Another excellent source for analytical pigment data is the Artists' Pigments series (Feller 1986; Roy 1993; Fitzhugh 1997) published by the National Gallery of Art, which covers a wide range of historic pigments. 4 4. IMAGE DATA FILESDigital video microscopy allows the conservator to create image data files as a future reference source. The conservator can create image data files for fibers, pigments, or any other type of specimen that may prove practical in performing side-by-side comparative analysis. Image data files can be created to suit the individual needs and purposes of the conservator. They can be created for exclusive in-house use or so they can be accessed by others, through a computer network, for instance. Images may be captured and saved in different image file formats, such as Tagged Image File (TIFF) and Joint Photographic Experts Group (JPEG), which were both developed by the Joint Photographic Experts Group (D'Amato and Klopfenstein 1996). Most video capture cards support these formats. Each storage file format has its own advantages and disadvantages, so the conservator should choose carefully from the options before setting up his or her own image file database. Concerns may involve issues about portability, storage, image stability, system integrity, accessibility, etc. Some common storage formats are Graphics Interchange Format (GIF), TIFF, Photo CD, and BitMaP (BMP) (D'Amato and Klopfenstein 1996). 5 5. CONCLUSIONSDigital video microscopy is a valuable tool for such purposes as pretreatment testing, identifying materials, and creating image data reference files. Its affordable cost and ease of use make it a very attractive addition to almost any existing photomicroscopy system that is equipped with a video camera by installing a video capture card and graphic software. The video capture card has the additional advantage of creating real-time movies, which are valuable for recording the physical behavior characteristics of various materials as well as for training purposes. In the future, advances in hardware and software digital technology will make digital video microscopy even more powerful, flexible, and affordable for the conservator. The several examples of practical applications of digital video microscopy highlighted here can only be expanded and enhanced as the process becomes more widely used over the broad range of specializations in conservation. ACKNOWLEDGEMENTSThe author would like to acknowledge the great work of Marc Reeves, New York Public Library Conservation Department, that has inspired the author's interest in digital video microscopy. The author also thanks very much Ersev Erdogan, Princeton Class of 1999 and now with Microsoft; Michele Hamill, Cornell University Preservation Department; and Stuart Kohler, Norwich University Computer Systems Department, for reviewing the drafts of this paper. I would also like to thank the reviewers and editors of JAIC. APPENDIXAPPENDIXThe digital video microscopy system used in the Special Collections Conservation Unit of Princeton University Library includes: Computer Power Mac G4/400MHz, 256 MB RAM Operating System Mac OS 9 Computer Monitor Apple 17 in., .25 dot pitch color monitor CCD Camera Javelin JE133 Digital Video Capture Card Aurora Fuse, 9 MB capture rate Polarized Light Microscope Olympus BH-2 Stereo Binocular Microscope Zeiss OpMI 1-FC REFERENCESBiggs, J.1994. Analysis and treatment of a painting on vellum with the aid of a video-microscopy system. In Book and Paper Group annual, vol. 14. Washington, D.C.: American Institute for Conservation. 1–7. C�t�, W. A.1980. Papermaking fibers: A photomicrographic atlas. Syracuse, N.Y.: Syracuse University Press. D'Amato, P. D., and R. C.Klopfenstein. 1996. Requirements and options for the digitalization of the illustration collections of the National Museum of Natural History. Washington, D.C.: Smithsonian Institution. Feller, R. L.1986. Artists' pigments: A handbook of their history and characteristics, vol. 1. Washington, D.C.: National Gallery of Art. Fitzhugh, E. W.1997. Artists' pigments: A handbook of their history and characteristics, vol. 3. Washington, D.C.: National Gallery of Art. Greenberg, A. D., and S.Greenberg. 1995. Digital images: A practical guide. Berkeley, Calif.: McGraw-Hill. Groce, G. C., and D. H.Wallace. 1957. The New York Historical Society's dictionary of artists in America, 1564–1860. New Haven, Conn.: Yale University Press. Kabir, I.1996. High performance computer imaging. Greenwich, Conn.: Manning Publications Co. Luther, A. C.1991. Digital video in the PC environment. 2d ed.New York: McGraw-Hill. McCrone, W. C.1982. The microscopical identification of pigments. Journal of the International Institute for Conservation—Canadian Group7(1 & 2):11–34. McCrone, W. C., L. C.McCrone, and J. G.Delly. 1995. Polarized light microscopy. 9th printing. Chicago, Ill.: McCrone Research Institute. Poynton, C. A.1996. A technical introduction to digital video. New York: John Wiley & Sons. Reilly, J. M.1986. The care and identification of 19th-century photographic prints. Rochester, N.Y.: Eastman Kodak Company. Robertson, J.1992. Forensic examination of fibers. Chichester, England: Ellis Horwood Limited. Roy, A.1993. Artists' pigments: A handbook of their history and characteristics, vol. 2. Washington, D.C.: National Gallery of Art. Bell, L. A.1990. Plant fibers for papermaking. McMinneville, Ore.: Liliaceae Press. Besser, H., and J.Trant. 1995. Introduction to imaging: Issues in constructing an image database. Santa Monica, Calif.: Getty Art History Information Program. Brown, C. W., and B. J.Shepherd. 1995. Graphics file formats, reference and guide. Greenwich, Conn.: Manning Publications Co. Cardullo, R. A., and E. J.Alm. 1998. Introduction to image processing. In Methods in cell biology, vol. 56, ed. G.Sluder and D. E.Wolf. San Diego, Calif.: Academic Press. Crown, D. A.1968. Forensic examination of paints and pigments. Springfield, Ill.: Charles C. Thomas. Feeley, J.1996. Digital video gets PCI religion. Macworld. February. 46–47. Huesmann, M.1999. Reading the world's secrets: 150 years of Leica microscope optics for progress in science and industry. Wetzlar, Germany: LMS Holdings GmbH. Inou�, T., and N.Gliksman. 1998. Techniques for optimizing microscopy and analysis through digital image processing. In Methods in cell biology, vol. 56, ed. G.Sluder and D. E.Wolf. San Diego, Calif.: Academic Press. Mayer, D.1994. Fiber identification (draft). In Paper conservation catalog, vol. 1. Washington, D.C.: American Institute for Conservation. Salmon, E. D.1998. A high-resolution multimode digital microscope system. In Methods in cell biology, vol. 56, ed. G.Sluder and D. E.Wolf. San Diego, Calif.: Academic Press. SOURCES OF MATERIALSCCD camera and microscopesNY/NJ Scientific P.O.Box 5155 Middlebush, N.J. 08875 ChemicalsJ.T. Baker A division of Mallinckrodt Baker Phillipsburg, N.J. 08865 Computer and monitorApple Computer 1 Infinite Loop Cupertino, Calif. 95014–6299 Digital video capture cardClubMac 7 Hammond Irvine, Calif. 92618 Fiber reference setsMcCrone Accessories 850 Pasquinnelli Drive Westmont, Ill. 60559–5531 Institute of Paper Science and Technology 500 10th Street, NW Atlanta, Ga. 30318 Japanese paperHiromi Paper 2525 Michigan Ave. #G-9 Santa Monica, Calif. 90404 Microscopes (Polarized light)NY/NJ Scientific P.O. Box 5155 Middlebush, N.J. 08875 McCrone paint pigment sample setMcCrone Accessories 850 Pasquinnelli Drive Westmont, Ill. 60559–5531 Wheat starch pasteTalas 568 Broadway New York, N.Y. 10012 AUTHOR INFORMATIONTED STANLEY has been the head of the Special Collections Conservation Unit and a paper conservator for Princeton University Library since 1992. From 1976 to 1992, he was a senior paper conservator at the Library of Congress in Washington, D.C. He has also studied paper conservation through the National Endowment for the Arts Fellowship for Museum Professionals at the Biblioth�que Nationale, Paris, and the Centro Nationale de Conservacion y Restauracion de Bien Culturales in Madrid, from 1984 to 1985. Address: Preservation Office, Firestone Library, Princeton University, One Washington Rd., Princeton, N.J. 08544–2098 Received for review March 16, 1999. Revised manuscript received November 5, 1999. Accepted for publication November 23, 1999. NOTES1.. The 1868 print had been mounted on a solid-ground wood board and was previously repaired with lengths of transparent pressure-sensitive tape, which had discolored and stained the print. Subsequently, the print was conserved by the following steps: mechanically removing the mount; removing the tape carrier, reducing the adhesive residue and stain in several baths of ethyl acetate; suction table washing with a (90:10) solution of absolute ethanol and carbon-filtered water spray; and a stretch-lining using double layers of tengujo Japanese paper and ethanol-saturated wheat starch paste. The print was pre-wetted with a (90:10) solution of absolute ethanol and carbon-filtered water spray before lining. The conservation treatment did not adversely affect the print in any way.The 1890 print was not mounted. It had severe planar distortion that could not be treated effectively. Severe skinning of the paper support at its verso was reinforced with tengujo Japanese paper and wheat starch paste.
 Section Index Section Index |