ASEPTIC TECHNIQUE: A GOAL TO STRIVE FOR IN COLLECTION RECOVERY OF MOLDY ARCHIVAL MATERIALS AND ARTIFACTSMARY-LOU E. FLORIAN
2 2. THE CAUSATIVE FUNGI2.1 2.1 LIFE CYCLE OF THE CONIDIAL FUNGITo design a logical aseptic technique, we must first have a general understanding of what we are attempting to control. The majority of fungal infestations that are problems in our museum collections are caused by a small group of fungi called the conidial fungi (Florian 1997). These fungi grow on surfaces of artifacts or archival materials and reproduce asexually by producing hundreds of conidia, which easily become airborne. It is the airborne conidia that are the major concern in collection recovery. The life cycle starts with a conidium (often called a spore) that germinates and produces a filament (hypha), which grows and branches in all directions to form a mass, collectively called the mycelium or colony. Colony formation only occurs if there are sufficient nutrients and moisture in the substrate. If growth is restricted by lack of moisture or of nutrients, a sparse, wispy growth may be present on surfaces. When the mycelium is mature, specialized hyphae called conidiophores are formed. On the conidiophores, thousands of conidia are produced, which can easily become airborne because of their small size (2–10 μm) and buoyancy. Our concern is the conidia. 2.2 2.2 THE ORIGIN OF FUNGAL SPECIESAirborne conidia make up the major part of an array of airborne particles that might also include hyphal fragments, pollen grains, and small particulate material. This mixture of airborne material is called the airspora. Outdoors, the airspora is monitored to determine high pollen or conidial counts that initiate a hay fever alert. Our homes and buildings are monitored to determine high conidial counts that may indicate a fungal problem. The fungal conidia commonly present in analyses of airspora are a cosmopolitan group of fungal species. The original source of these species is outdoor decaying plant materials. The commonest species (spp.) are in the genera Alternaria, Cladosporium, Aspergillus, and Penicillium. The same fungal species are present in airspora outdoors and indoors, but in different relative amounts. In outdoor air Alternaria spp. and Cladosporium spp. are more common because outdoors there are more amplifiers (sites of active growth) on plant materials for these species. Indoors we produce more amplifiers for Penicillium spp. and Aspergillus spp. in, for example, food preparation and storage areas, microenvironments such as storage boxes on cement floors or against cold metal framing of windows, and faulty humidification equipment and air conditioning ducts. Table 1 collates information from a review of conservation literature (Florian 1994) about the viable conidia cultured from surfaces of different types of artifacts and archival material worldwide. The species are shown to be cosmopolitan; they are not substrate specific—that is, they do not grow on only one type of material. Basically, this type of analysis shows the variety of species that landed on the surface of the artifact or archival material or were incorporated into it during its manufacturing and were still viable at the time of sampling.
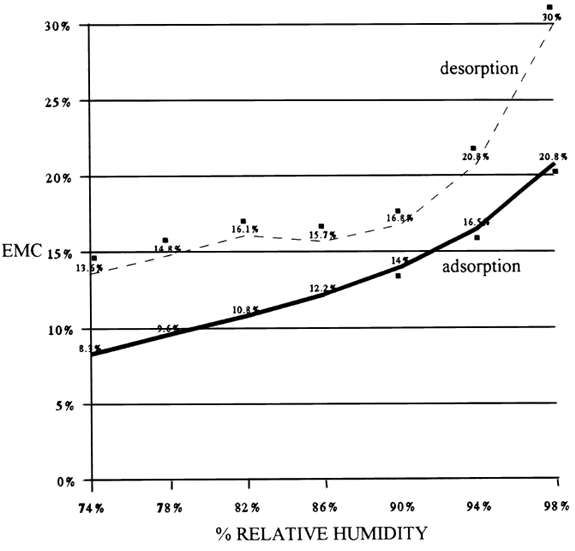
While fungal species may not be causing a problem, they are contaminants. It is important to note that the four most common species are on all types of artifacts. 2.3 2.3 THE CONIDIUM2.3.1 2.3.1 The Structure of the ConidiumThe conidia of the conidial fungi are usually spherical structures about 2–10 μm in diameter. They are a single cell of low metabolic activity with a reproductive and survival function. They are the main unit of dispersal. The conidium has a rigid, water impermeable, nonliving, protective outer cell wall that surrounds the living protoplast of the cell. Between the cell wall and the protoplast is hygroscopic material that adsorbs water when the cell wall becomes permeable and germination is initiated. The protoplast contains cytoplasm filled with many different types of organelles—e.g., nucleus, mitochondria, and endoplasmic reticulum—required to run the metabolism of the cell. The cytoplasm also contains all the material needed for its initial development—genetic material, food (as lipid droplets, carbohydrates, minerals, and protein), and a water solution consisting largely of enzymes. The allergenic or toxic substance is usually produced in the cell wall of the conidia and hyphae. The cell wall has characteristic markings that are often species specific and aid in the identification of the fungal species. What we are worried about in collection recovery is the movement of the conidia from moldy materials into the air. 2.3.2 2.3.2 The Conidium as a Health HazardConidia do not always present a health hazard. Under normal conditions or a chance exposure, the inhalation of the airborne conidia does not cause any response in most people, although there are a few who are hyperallergic and may have an allergic response from the inhalation of this low number of conidia. On the other hand, continuous inhalation of or skin exposure to conidia over a long period of time and in large quantities may cause skin or eye irritation, allergic reactions, respiratory problems, and pathological problems (Health Canada 1995). Only a few fungal species produce toxins that can cause severe respiratory problems and related complications. Such species usually require a special environment to grow. For example, Stachybotrys atra requires cellulosic materials that are wet over a long time, such as wet drywall behind a vapor barrier. It is important to know that the allergenic or toxic substance of conidia and hyphae is active in both dead and living material. For example, tests on Stachybotrysatra toxin show that it is still active after the conidia and hyphae have been killed by autoclaving or being dried and fragmented by acoustic vibrations (Sorenson et al. 1987). 2.3.3 2.3.3 The Conidium as a Hazard to Artifacts and Archival Materials (Objects)Besides posing a potential health hazard, airborne conidia may land on the surface material of adjacent artifacts and contaminate them with conidia, thus posing a threat of future infestations. The fungal filaments do not adsorb water vapor from the air. They adsorb water from the materials through the very tips of the hyphae, where the water-impermeable cell wall has not yet been completely formed. The water in materials is influenced by relative humidity, the material's physical characteristics (e.g., porosity and surface areas), and the chemicals in the materials (e.g., salts, tannins, glycerol, sugars, etc.). If the moisture content in the material is appropriate, the conidia, if viable and activated, may germinate. The prerequisite to cleaning moldy materials is that they must be dry. Materials that have been wet and are in the process of drying—desorption—may have more water in them than we expect. Figure 1 shows that the amount of water in leather at the same relative humidity (RH) and temperature will vary depending on whether the material has adsorbed water or desorbed water. This differential, called the hysteresis curve, is common to all organic materials. It means that collection materials that have been wet and are dried in the parameters of their original environment will contain more water than they originally did before they became wet. In Figure 1, for example, at 74% RH the leather contained 5.3% more moisture on desorption than on adsorption.
The most important point to understand from Figure 1 is that the leather on adsorption did not support fungal growth until it had 14% equilibrium moisture content (EMC), which was obtained at 90% RH, whereas on desorption the leather may support fungal growth at 74% RH because it contains close to 14% EMC. Unfortunately, this experiment did not go below 74% RH, but the point it illustrates is clear: it is not RH that controls the growth of fungi on materials; it is the water in the materials. Thus cessation of weight loss may not be a safe guide to dryness. Moisture content of the materials is a better guide, but the true standard for comparison should be materials that have not been wet and have been at equilibrium with the environment for a long period. Yet this information is not always available. If it is not, it is advisable to dry wet collection objects at a lower RH or a higher temperature than in their original environment, as either method will lower the EMC. Materials with a high regain such as thick, loosely felted paper pose a particular problem, but we must be aware of this phenomenon and consider it with all materials. |