ASEPTIC TECHNIQUE: A GOAL TO STRIVE FOR IN COLLECTION RECOVERY OF MOLDY ARCHIVAL MATERIALS AND ARTIFACTSMARY-LOU E. FLORIAN
ABSTRACT—ABSTRACT—In a collection recovery of moldy heritage materials, it is impossible to attain a completely aseptic technique because of the open sites and often large numbers of objects. Still, it is a goal to strive for. Aseptic technique can prevent cross-contamination from moldy to nonmoldy objects and an increase in airborne conidia in the work site, reducing health hazards as well as hazards to objects. Personal protection—masks, gloves, and garments—and a method for moving air away from the conservator during recovery treatments must be used. Another reason for using aseptic technique is that monitoring methods for determining contamination of air or surfaces have biases that make the results difficult if not impossible to interpret; thus every effort to prevent contamination is essential. TITRE—Techniques aseptiques: un but � atteindre lors de la r�cup�ration des collections d'objets et de documents d'archives moisis. R�SUM�—Lors de la r�cup�ration de collections patrimoniales qui ont �t� envahies par les moisissures, il est impossible de parvenir � conserver des techniques compl�tement aseptiques, car souvent les op�rations ont lieu dans des endroits ouverts sur l'ext�rieur et � cause du grand nombre d'objets � traiter. Des techniques aseptiques peuvent pourtant emp�cher le transfer de moisissures aux objets qui n'y ont pas encore �t� expos�s et diminuer le nombre de conidies dans l'aire de travail, ce qui r�duit les risques de contamination du personnel et des objets. Du mat�riel de protection personnelle, tels des respirateurs, gants et v�tements protecteurs, doit �tre utilis�, ainsi que des m�thodes pour �loigner l'air contamin� de l'endroit o� travaillent les restaurateurs lors des op�rations de r�cup�ration. Une autre raison pour employer des techniques aseptiques est la difficult�, sinon l'impossibilit�, d'interpr�ter correctement les r�sultats des analyses qui servent � d�terminer la pr�sence de contaminants dans l'air ou sur les surfaces. Ainsi un probl�me de moisissures pourrait �tre pr�sent sans toutefois �tre d�tect�. TITULO—Tecnica aseptica: una meta hacia la cual encaminarse en la recuperaci�n de material de archivo y objetos mohosos. RESUMEN—En la recuperaci�n de una colecci�n de objetos patrimoniales mohosos, es imposible lograr t�cnicas completamente as�pticas debido a que los lugares son abiertos y con mucha frecuencia hay una gran cantidad de objetos. Sin embargo, esta es una meta que se debe tener. Las t�cnicas as�pticas puede prevenir inter contaminaci�n entre objetos mohosos y los que no lo estan, y un aumento de conidia que es transportada en el aire en el sitio de trabajo, reduciendo asi tanto los peligros que atentan contra la salud, como peligros para los objetos. Se deben utilizar mascaras de protecci�n personal, guantes y vestidos, y un sistema para mover el aire de modo que se aleje del conservador cuando esta llevando a cabo los tratamientos de recuperaci�n. Otra raz�n para utilizar t�cnicas as�pticas es que los m�todos de seguimiento para determinar la contaminaci�n del aire y las superficies son sesgadas haciendo que los resultados sean dificiles, sino imposibles, de interpretar de manera tal que un problema de contaminaci�n mic�tica puede estar presente pero no ser detectado. 1 1. INTRODUCTIONWhen one is working with pure cultures of strains of fungi in mycological research, it is essential that the cultures not be contaminated by airborne conidia or by nonsterile tools or materials used in the culturing technique. This essential condition is accomplished by an aseptic technique that involves the use of sterile materials and procedures in all activities. If toxic fungi are involved, masks, garments, gloves and a fume hood in which the air is drawn away from the worker into high-efficiency particulate air (HEPA) filters will reduce health hazards. A type 2 laminar flow hood is often used. But when a conservator is confronted with the recovery of a moldy heritage collection or group of objects, many issues have to be considered. We are directed in our collection recovery treatments and procedures by professional ethics and our knowledge of the objects' materials, preciousness, and fragility. We are also concerned about health issues and cross-contamination of objects. Although it is impossible to attain a completely aseptic technique because of open sites and often large numbers of objects, the aseptic technique is a great advance in conservation methods and something to strive for. As conservators, our goals are to prevent fungal cross-contamination from moldy to nonmoldy objects, to reduce future fungal problems, to obtain successful decontamination and cleaning of the object, and to prevent an increase in the level of airborne conidia in the work site so as to reduce health hazards and hazards for other objects. 2 2. THE CAUSATIVE FUNGI2.1 2.1 LIFE CYCLE OF THE CONIDIAL FUNGITo design a logical aseptic technique, we must first have a general understanding of what we are attempting to control. The majority of fungal infestations that are problems in our museum collections are caused by a small group of fungi called the conidial fungi (Florian 1997). These fungi grow on surfaces of artifacts or archival materials and reproduce asexually by producing hundreds of conidia, which easily become airborne. It is the airborne conidia that are the major concern in collection recovery. The life cycle starts with a conidium (often called a spore) that germinates and produces a filament (hypha), which grows and branches in all directions to form a mass, collectively called the mycelium or colony. Colony formation only occurs if there are sufficient nutrients and moisture in the substrate. If growth is restricted by lack of moisture or of nutrients, a sparse, wispy growth may be present on surfaces. When the mycelium is mature, specialized hyphae called conidiophores are formed. On the conidiophores, thousands of conidia are produced, which can easily become airborne because of their small size (2–10 μm) and buoyancy. Our concern is the conidia. 2.2 2.2 THE ORIGIN OF FUNGAL SPECIESAirborne conidia make up the major part of an array of airborne particles that might also include hyphal fragments, pollen grains, and small particulate material. This mixture of airborne material is called the airspora. Outdoors, the airspora is monitored to determine high pollen or conidial counts that initiate a hay fever alert. Our homes and buildings are monitored to determine high conidial counts that may indicate a fungal problem. The fungal conidia commonly present in analyses of airspora are a cosmopolitan group of fungal species. The original source of these species is outdoor decaying plant materials. The commonest species (spp.) are in the genera Alternaria, Cladosporium, Aspergillus, and Penicillium. The same fungal species are present in airspora outdoors and indoors, but in different relative amounts. In outdoor air Alternaria spp. and Cladosporium spp. are more common because outdoors there are more amplifiers (sites of active growth) on plant materials for these species. Indoors we produce more amplifiers for Penicillium spp. and Aspergillus spp. in, for example, food preparation and storage areas, microenvironments such as storage boxes on cement floors or against cold metal framing of windows, and faulty humidification equipment and air conditioning ducts. Table 1 collates information from a review of conservation literature (Florian 1994) about the viable conidia cultured from surfaces of different types of artifacts and archival material worldwide. The species are shown to be cosmopolitan; they are not substrate specific—that is, they do not grow on only one type of material. Basically, this type of analysis shows the variety of species that landed on the surface of the artifact or archival material or were incorporated into it during its manufacturing and were still viable at the time of sampling.
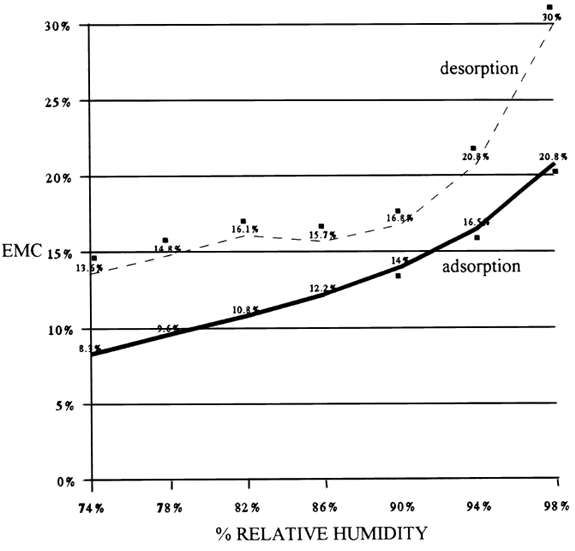
While fungal species may not be causing a problem, they are contaminants. It is important to note that the four most common species are on all types of artifacts. 2.3 2.3 THE CONIDIUM2.3.1 2.3.1 The Structure of the ConidiumThe conidia of the conidial fungi are usually spherical structures about 2–10 μm in diameter. They are a single cell of low metabolic activity with a reproductive and survival function. They are the main unit of dispersal. The conidium has a rigid, water impermeable, nonliving, protective outer cell wall that surrounds the living protoplast of the cell. Between the cell wall and the protoplast is hygroscopic material that adsorbs water when the cell wall becomes permeable and germination is initiated. The protoplast contains cytoplasm filled with many different types of organelles—e.g., nucleus, mitochondria, and endoplasmic reticulum—required to run the metabolism of the cell. The cytoplasm also contains all the material needed for its initial development—genetic material, food (as lipid droplets, carbohydrates, minerals, and protein), and a water solution consisting largely of enzymes. The allergenic or toxic substance is usually produced in the cell wall of the conidia and hyphae. The cell wall has characteristic markings that are often species specific and aid in the identification of the fungal species. What we are worried about in collection recovery is the movement of the conidia from moldy materials into the air. 2.3.2 2.3.2 The Conidium as a Health HazardConidia do not always present a health hazard. Under normal conditions or a chance exposure, the inhalation of the airborne conidia does not cause any response in most people, although there are a few who are hyperallergic and may have an allergic response from the inhalation of this low number of conidia. On the other hand, continuous inhalation of or skin exposure to conidia over a long period of time and in large quantities may cause skin or eye irritation, allergic reactions, respiratory problems, and pathological problems (Health Canada 1995). Only a few fungal species produce toxins that can cause severe respiratory problems and related complications. Such species usually require a special environment to grow. For example, Stachybotrys atra requires cellulosic materials that are wet over a long time, such as wet drywall behind a vapor barrier. It is important to know that the allergenic or toxic substance of conidia and hyphae is active in both dead and living material. For example, tests on Stachybotrysatra toxin show that it is still active after the conidia and hyphae have been killed by autoclaving or being dried and fragmented by acoustic vibrations (Sorenson et al. 1987). 2.3.3 2.3.3 The Conidium as a Hazard to Artifacts and Archival Materials (Objects)Besides posing a potential health hazard, airborne conidia may land on the surface material of adjacent artifacts and contaminate them with conidia, thus posing a threat of future infestations. The fungal filaments do not adsorb water vapor from the air. They adsorb water from the materials through the very tips of the hyphae, where the water-impermeable cell wall has not yet been completely formed. The water in materials is influenced by relative humidity, the material's physical characteristics (e.g., porosity and surface areas), and the chemicals in the materials (e.g., salts, tannins, glycerol, sugars, etc.). If the moisture content in the material is appropriate, the conidia, if viable and activated, may germinate. The prerequisite to cleaning moldy materials is that they must be dry. Materials that have been wet and are in the process of drying—desorption—may have more water in them than we expect. Figure 1 shows that the amount of water in leather at the same relative humidity (RH) and temperature will vary depending on whether the material has adsorbed water or desorbed water. This differential, called the hysteresis curve, is common to all organic materials. It means that collection materials that have been wet and are dried in the parameters of their original environment will contain more water than they originally did before they became wet. In Figure 1, for example, at 74% RH the leather contained 5.3% more moisture on desorption than on adsorption.
The most important point to understand from Figure 1 is that the leather on adsorption did not support fungal growth until it had 14% equilibrium moisture content (EMC), which was obtained at 90% RH, whereas on desorption the leather may support fungal growth at 74% RH because it contains close to 14% EMC. Unfortunately, this experiment did not go below 74% RH, but the point it illustrates is clear: it is not RH that controls the growth of fungi on materials; it is the water in the materials. Thus cessation of weight loss may not be a safe guide to dryness. Moisture content of the materials is a better guide, but the true standard for comparison should be materials that have not been wet and have been at equilibrium with the environment for a long period. Yet this information is not always available. If it is not, it is advisable to dry wet collection objects at a lower RH or a higher temperature than in their original environment, as either method will lower the EMC. Materials with a high regain such as thick, loosely felted paper pose a particular problem, but we must be aware of this phenomenon and consider it with all materials. 3 3. SAMPLING OF COLLECTION RECOVERY SITES FOR THE LEVEL OF CONIDIAL CONTAMINATION3.1 3.1 SAMPLING GOALSThe goals of sampling or monitoring for fungal contamination in a building are: (1) to determine if there is a sufficient number or level of conidia to be considered a problem; (2) to identify the species of the fungal conidia to discover if they are a health hazard; and (3) to assist in the location of the source (amplifier) of the conidia. 3.2 3.2 SAMPLING METHODSThe surface sampling of moldy materials for species identification will be done prior to any recovery activity. We know that the moldy objects are the amplifier; thus the concern here is the change in the level of contamination at the recovery site. 3.2.1 3.2.1 MethodsA variety of different methods of air and surface sampling can ascertain conidial contamination. The commonest methods are described and critiqued in a report by Health Canada (1995). Essentially, air sampling may be done by pumping a measured amount of air onto or through a collecting device or by sedimentation (relying on gravity) of the conidia onto settle plates. In both cases the captured conidia are cultured on a growth media, and the results are recorded as colony forming units: CFU/volume of air or CFU/time. Other methods of air sampling involve the capturing of conidia by pumping air or sedimentation directly onto microscope slides for microscopic identification and counting. These methods do not involve the culturing of the trapped conidia. The results of these methods are recorded as total spore count-nonviable (TSC/volume of air or TSC/time). Even though the term “spore” is used, it is conidia that are counted. Surface sampling is often done by using sterile cotton swabs to remove conidia from a specific surface area. The swabs may be covered with a nutrient medium, or plain swabs may be washed in a nutrient medium. The nutrient medium is then cultured, and the swab analysis is recorded in CFU/surface area. Surface sampling is also done by using Scotch tape to remove conidia in dust from contaminated surfaces for microscopic examination and recorded as TSC/surface area. 3.2.2 3.2.2 Biases in CFU TestsResults recorded as CFU/volume of air, time, or surface area are inevitably biased. First, because the methods involve only conidia that can germinate and develop subsequent growth, false negatives may occur because some conidia may be dead, not activated and unable to germinate, or unable to utilize the growth medium used. Using several growth media can alleviate some of this bias. Health Canada (1995) suggests that microscopy should be done along with CFU determinations to detect the excluded conidia. Second, air pumping and sedimentation methods produce a bias because air samples are not homogeneous. The air sampled may involve still air, air drafts, air conditioning air, or air disturbed by human activity. Third, the settling rate of conidia onto surfaces depends on their weight, size, morphology, and water content. Thus a species with small and light conidia may be in low amounts or excluded from the sedimentation sample while another species with large conidia will be present in biased high amounts. The common Aspergillus and Penicillium species, which are our major concern, are poor settlers because they are small and light. 3.2.3 3.2.3 Biases in TSC/Volume of Air TestsResults recorded as TSC/volume of air are like wise biased by nonhomogeneous air and settling rates of conidia. Yet the microscopic analysis results give a total of all conidia captured, eliminating the bias due to false negatives. Additionally, staining of conidia with a vital stain can be used to determine if they are viable. The pumping air method with TSC/volume of air gives the most accurate analysis of air (MacRae 1998). 3.2.4 3.2.4 Bias in Surface SamplingSurface sampling with Scotch tape or swabs removes all conidia present, i.e., the deposition of conidia over an unknown period of time. Yet, conidia that have settled on the surface over years may have nothing to do with the collection recovery activities. In collection recovery it is essential to first test a cleaned surface to obtain a reference point that can be compared to samples taken during recovery activities. 3.3 3.3 INTERPRETATION OF TEST RESULTSThe biases described above limit our ability to determine when we have a fungal growth problem. Health Canada (1995) points out that even if conidia concentrations in room air were known with absolute accuracy, we would be little further ahead in understanding the association between these numbers and allergic or respiratory symptoms. In addition essential dose-response information to correlate numbers of fungal conidia of particular fungal species to health effects in humans is absent for all molds, and as tolerances in individuals vary so greatly, acceptable dose-response data continue to be very difficult to obtain. Because of the biases in sampling and the difficulty in the assessment of the problems, I have come to the conclusion that the only logical approach during cleaning of moldy archival material or artifacts in collection recovery sites is to strive for aseptic technique, a preventive approach. 4 4. ASEPTIC TECHNIQUE: A LOGICAL PREVENTION OF FUNGAL PROBLEMSAseptic technique uses procedures that prevent fungal cross-contamination from moldy to nonmoldy objects, thereby reducing future fungal problems. The technique also prevents an increase in the level of airborne conidia in the work site, thereby reducing health hazards and hazards to other objects. The recovery process includes initial storage and/or treatment, dehydration of the wet objects, and then decontamination of the dry objects. 4.1 4.1 INITIAL STORAGE AND TREATMENT, INCLUDING DEHYDRATIONThe initial storage and/or treatment may be at the disaster site, or at another site, or in cold storage or freezing facilities. Aseptic technique must be considered in all activities: the packing and unpacking techniques, transportation process, protection of objects that are just wet and not moldy, drying methods such as air movement or exposing surfaces, and facilities maintenance. Drying method may involve air flow, dehumidification, heat (less than 37�C), and freeze-drying. The pros and cons of the different storage facilities and drying methods are discussed by Mary-Lou Florian (1997). 4.2 4.2 DecontaminationDecontamination reduces the conidia numbers, thereby reducing health hazards and the potential for future fungal problems on the objects. Decontamination involves two steps: the killing and the removal of the conidia and the mycelia. Drying the objects will kill the hydrated mycelia and conidia but not dormant, nonactivated dry conidia. Therefore the conidia and mycelia must be removed, even if they are dead. In exceptional cases where a toxic fungus has contaminated materials, killing all the fungal structures is essential prior to cleaning to prevent the spread of viable conidia, but the fungal structures still need to be removed to reduce the health hazard. Decontamination methods include surface cleaning by vacuuming, electrostatic dusting, brushing, erasing, and dry-sponging. Because there is little literature on the success of these methods, it is essential that, prior to object treatment, the methods are tested for their efficiency. They must also be assessed in reference to aseptic technique. A normal background of airborne conidia will be present at every collection recovery site, but the number of conidia can increase dramatically in the presence of moldy objects. Using aseptic techniques can drastically reduce their presence. We cannot hope to eliminate all the conidia, however, because as soon as cleaned objects are placed in air, they become contaminated with conidia from normal airspora. The greatest threat to mold-free objects or objects that have been decontaminated is cross-contamination from moldy objects, contaminated materials, and airborne conidia. Contaminated materials include anything that may have come in contact with the moldy objects, such as packing cases or boxes, packing materials, gloves, brushes, and other tools used during cleaning, and even the surfaces they are put on for cleaning. 4.3 4.3 ASEPTIC TECHNIQUE STEPSMost aseptic technique steps are simple and logical, but they require a new awareness in our procedures. The following is a preliminary list of steps to improve aseptic technique:
Every collection recovery is unique, so methods and procedures will be specific to each situation, but the philosophy of aseptic technique should direct these methods or procedures. 5 5. CONCLUSIONSWe cannot rely on monitoring or sampling methods to determine if we are preventing fungal hazards to objects or ourselves. Thus we must strive for aseptic technique as a preventive approach. REFERENCESFlorian, M-L. E.1994. Conidial fungi (mould, mildew) biology: A basis for logical prevention, eradication and treatment for museum and archival collections. Leather Conservation News10:1–26. Florian, M-L. E.1997. Heritage eaters: Insects and fungi in heritage collections. London: James & James (Science Publishers) Ltd. Health Canada. 1995. Sampling methods for fungal bioaerosols and amplifiers in cases of suspected indoor mould proliferation. Fungal contamination in public buildings: A guide to recognition and management. Tunney's Pasture, Ottawa, Ontario, Canada: Environmental Health Directorate, Health Canada. App. D. MacRae, J.1998. Personal communication. John MacRae & Associates Inc., Victoria, British Columbia, Canada. Rose, C. D., and J. N.Turner. 1951. Mold growth on leather as affected by humidity changes. Journal of the Society of Leather Trades' Chemistry35:37–45. Sorenson, W. G., D. G.Frazer, B. B.Jarvis, J.Simpson, and V. A.Robinson. 1987. Trichothecene mycotoxins in aerosolized conidia of Stachbotrys atra. Applied and Environmental Microbiology47:1370–75. AUTHOR INFORMATIONMARY-LOU FLORIAN worked as a contract biologist and conservation scientist at Canadian Conservation Institute in Ottawa, Ontario, Canada, from 1972 to 1978. She then worked as a conservation scientist at the Royal British Columbia Museum, retiring as head of conservation services in 1991. She currently has a consulting business and does volunteer work for the museum in her capacity of research associate emerita. She is also doing research on fungal stains in old books and is writing a series of teaching manuals on organic materials as a Samuel H. Kress Foundation Fellow. Florian has a master's degree in botany (plant anatomy) from the University of Texas and has completed university courses in fine art and ethnology. For her undergraduate honors research project she specialized in mycology. She worked at B.C. Research Institute after graduation for three years, completing research and publishing an article in microbiology. Since then, she has taught and published on many aspects of conservation and the identification of organic materials, insect and fungal pest control, prevention and eradication, natural history specimens, and the biology of fungi in the museum environment. She is an Honorary Member of AIC and was awarded a 125th Commemorative Medal from the Governor General of Canada for her contribution to preserving her community heritage, a British Columbia Museums Association Distinguished Service Award, and a Society for the Preservation of Natural History Collections Lifetime Achievement Award. Address: 129 Simcoe St., Victoria, British Columbia, Canada V8V 1K5.
 Section Index Section Index |