ATOMIC OXYGEN TREATMENT AS A METHOD OF RECOVERING SMOKE-DAMAGED PAINTINGSSHARON K. RUTLEDGE, BRUCE A. BANKS, MARK FORKAPA, THOMAS STUEBER, EDWARD SECHKAR, & KEVIN MALINOWSKI
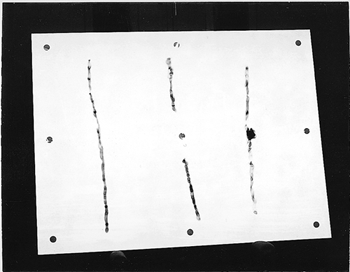
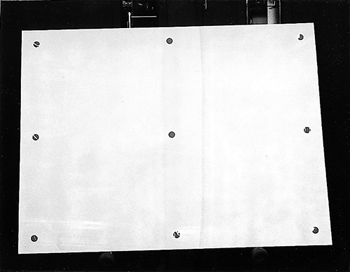
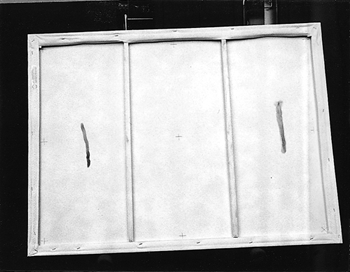
ABSTRACT—ABSTRACT—The noncontact technique that is described uses atomic oxygen, generated under low pressure in the presence of nitrogen, to remove soot and charred varnish from the surface of a painting. The process, which involves surface oxidation, permits control of the amount of surface material removed. The effectiveness of the process was evaluated by reflectance measurements from selected areas taken during the removal of soot from acrylic gesso, ink on paper, and varnished oil paint substrates. For the latter substrate, treatment also involved the removal of damaged varnish and paint binder from the surface. TITRE—L'oxyg�ne atomique: un traitement pour les peintures endommag�es par le feu. R�SUM�—La technique sans contact d�crite dans cet article fait appel � l'oxyg�ne atomique g�n�r� sous faible pression et en pr�sence d'azote pour enlever la suie et le vernis carbonis� de la surface d'une peinture. Le proc�d�, qui se base sur l'oxydation de la surface, permet de contr�ler la quantit� de mati�re qu'on enl�ve � la surface. On a �valu� l'efficacit� du proc�d� au moyen de mesures de r�flectance ponctuelles dans le cas de trois substrats recouverts de suie: du gesso acrylique, de l'encre sur papier et une peinture � l'huile vernie. Dans ce dernier cas, le traitement a aussi consist� � l'�limination superficielle du vernis et du liant endommag� par le feu. TITULO—El tratamiento con ox�geno at�mico como m�todo para la recuperaci�n de pinturas da�adas por humo. RESUMEN—La t�cnica sin contacto descrita aqui utiliza ox�geno at�mico generado a baja presi�n en presencia de nitr�geno para remover holl�n y barn�z chamuscado de la superficie de una pintura. El proceso, que incluye oxidaci�n superficial, permite controlar la cantidad de material de la superficie que es removido. La efectividad del proceso fue evaluada por medio de mediciones de la reflectividad en ar�as seleccionadas y fueron tomadas durante la remoci�n del holl�n depositado sobre yeso acr�lico, tinta sobre papel y barnices sobre sustratos de pintura al �leo. Sobre la pintura al �leo, el tratamiento incluy� la remoci�n de la superficie del barn�z da�ado y del aglutinande de pigmento. 1 1. INTRODUCTIONResearch into using atomic oxygen as a treatment technique started through inquiries by the Conservation Department of the Cleveland Museum of Art as to available NASA technologies that could be used to remove urethane varnish. Presentation of the results achieved with removal of various types of varnish, and discussion with conservators from other organizations, led to investigating the technique for removing soot and char from the surface of paintings and other fine art. Conservators believed such cleaning to be a problem area in need of some additional tools. Atomic oxygen is present in the earth's atmosphere at altitudes where satellites typically orbit. It has been shown to react chemically with surface coatings or deposits that contain carbon (Banks et al. 1988; Banks and Rutledge 1988). The reaction converts the carbon to carbon monoxide and some carbon dioxide. Water vapor is also a by-product of the reaction if the surface contains carbon-hydrogen bonds. Depending on the material's chemical structure, the reaction can proceed through hydrogen atom abstraction, oxygen addition to form excited radicals followed by hydrogen atom elimination, oxygen insertion into the C-H bonds, and replacement by formation of alkoxy radicals (Banks and Rutledge 1988; Dever 1991). The majority of the reaction products are volatile species. The rate of the reaction will vary with the surface chemistry, atomic arrangement, temperature, energy of the oxygen atoms, and presence of electrons and other species. Materials already in a high oxidation state, such as metal oxides, are not affected by atomic oxygen. Exposure to atomic oxygen can be harmful to a satellite if enough carbon-containing materials critical to its operation are removed. Due to the importance of the problem and the need to test potential solutions for protecting surfaces from reaction, facilities have been developed for producing atomic oxygen on Earth (Banks and Rutledge 1988; Banks et al. 1989). Radio frequency (RF) and microwave radiation or electron bombardment has been used to dissociate molecular oxygen into atomic oxygen. Either these atoms can be directed at a surface as a gentle flow of gas, or the surface can be immersed in the gaseous atomic oxygen. The exposure is typically performed in a vacuum chamber where pressures can range from 1.3 � 10−4 Pa (1.93 � 10−8 psi) to 13.3 Pa (1.93 � 10−3 psi), depending on the dissociation process used. This condition is defined as a rough vacuum and requires only a mechanical vacuum pump to achieve. The atomic oxygen reaction is confined to the surface because the oxygen atoms have a high reactivity, usually reacting with what is directly in their path on either first or second contact. Recombination into relatively inactive molecular oxygen becomes more likely with multiple collisions, so reactions deep into the surface are very rare unless a lot of large, straight, open pores extend deep into the material. Atomic oxygen is of interest for cleaning paintings because the process is in the gas phase, there is no mechanical contact, and the reaction is confined to the surface, which reduces the risk of damaging the underlying paint or canvas. The atomic oxygen cleaning technique has been demonstrated to be effective for removing soot from small sample sections of canvas, acrylic gesso, and an unvarnished oil painting (Rutledge and Banks 1996). This article investigates its usefulness in removing soot from acrylic gesso and ink on paper, and in removing soot and thermally damaged binder and varnish from an oil painting. The process, which has been patented by NASA, is intended not to be a replacement for conventional techniques but to be an additional tool for use where conventional techniques may not be effective (Banks and Rutledge 1996). 2 2. PROCEDURE2.1 2.1 TEST OBJECTSA commercially available artist's stretched canvas (cotton canvas primed with acrylic gesso, approximately 90 � 120 cm) was used to perform the initial test of the large-area atomic oxygen treatment system. The stretcher was held above a wax candle flame, and the candle was moved back and forth underneath the stretcher to create soot streaks on both the paintable surface and the back of the canvas. Two fire-damaged works of art were also used in testing the atomic oxygen cleaning technique. The first was a Roy Lichtenstein (b. 1923) ink drawing on paper (untitled abstract from 1950, approximately 21.6 � 27.9 cm). The work had been heavily smoke-damaged and partially thermally decomposed in a fire. The McKay Lodge Fine Arts Conservation Laboratory in Oberlin, Ohio, had previously tried float washing in alkaline water (8.0 pH ammonium hydroxide) for a few hours followed by immersion in a sodium borohydride solution (< 1% v/v), but these processes had a minimal effect on the appearance of the drawing. The second work was a copy of the Raphael (1483–1520) painting Madonna of the Chair. It was painted by Bianchini of the Studio Viale Petrarca in Florence, Italy, and it was originally displayed in St. Alban's Episcopal Church in Cleveland, until an arson fire destroyed the church in June 1989. This painting was given to the Cleveland Museum of Art for treatment. The Madonna painting, a varnished oil painting approximately 74.3 � 74.3 cm, was heavily smoke-damaged and partially charred. A section of the painting was initially treated at the museum with acetone, then with methylene chloride and some additional solvents. Some of the soot and varnish were removed by these techniques, but the surface was still very dark, and features were difficult to distinguish. The Madonna painting was considered to be unsalvageable and was donated for testing of the atomic oxygen treatment process. 2.2 2.2 APPARATUS FOR ATOMIC OXYGEN CLEANINGCleaning of the test objects was performed in a large vacuum chamber that could hold a stretched painting roughly 1.5 � 2.1 m. The vacuum chamber was not specifically designed to hold paintings, but was modified to be able to accommodate paintings of this size suspended in a vertical position inside. The vacuum in the chamber is provided by conventional mechanical vacuum pumps, which can be used with traps but for these tests were not. Pressures during treatment range from 0.13 Pa (1.93 � 10−5 psi) to 0.667 Pa (9.67 � 10−5 psi). Two large aluminum parallel plates inside the chamber are used as electrodes to create the atomic oxygen through radio frequency (RF) excitation. One plate is connected to an RF power supply manufactured by RF Power Products Inc. operating at roughly 400 watts; the second plate is at ground potential. From the ground plate protrude several bolts from which test objects to be cleaned can be suspended directly or by hanging with fine wire. The objects are hung so that the ground plate is in contact with the back of the object, thereby shielding the back side from the atomic oxygen during cleaning. A controlled entry of air into the chamber at rates between 50 and 280 standard cm3 per minute provides the source of the atomic oxygen. Radio-frequency oscillating voltage between the two plates causes dissociation of the molecular oxygen and nitrogen in the air into atomic species, creating a plasma discharge with a pink glow between the plates. The atomic nitrogen has been found not to have an effect on carbon removal, and to date no detrimental effects, such as changes in coloration, have been observed on any of the test objects because of its presence. An automated timer and NASA-designed and -constructed controller on the system allows the cleaning to proceed unattended over a desired time frame, and will turn the system off if a loss in vacuum, water cooling to the pumps and power supply, or a drop in plasma intensity is detected. 2.3 2.3 MONITORING AND ASSESSMENTMonitoring and assessment of the treatment process were performed by removing the canvases from the vacuum chamber and measuring in air the diffuse reflectance from selected portions of the test objects at periodic intervals in the cleaning process. A quartz halogen microscope light set at full intensity, with a color temperature of 3200 K (blackbody peak wavelength of 900 nm), was mounted on an aluminum beam so that the light could hit the surface of a painting at roughly a 45� angle. A photodiode detector was placed near the light source. The equipment was placed in a dark room to minimize effects from stray light. The reflectance from a magnesium oxide-coated glass slide was used to correct the data from the detector to eliminate drifts in the intensity of the light source between measurements. The area that could be illuminated was approximately 1.91 cm in diameter, so it was necessary to select areas from the test articles that were both uniform over this size range and had the potential for changing the most (largest contrast) during the cleaning process. In this way, the end point of the cleaning could be determined by looking for a leveling-off of the reflected light signal, indicating that no further change was taking place. 3 3. RESULTS AND DISCUSSION3.1 3.1 CLEANING OF SMOKE STREAKS FROM GESSOThe first test of the large-area treatment capability was performed on a prepared canvas that had been streaked on the front and back with soot from a wax candle flame as described in section 2.1. Figure 1a shows the front side of the prepared canvas with smoke streaks prior to atomic oxygen exposure. After approximately 14 hours of cleaning in atomic oxygen, the smoke streaks were completely removed from the gesso surface, as shown in figure 1b. The rear of the canvas remained untouched, as shown by the comparable photos of the back side of the canvas in figures 2a and 2b. This result demonstrated the ability of the system to uniformly remove soot over a large area and verified that the proximity of the back side to the plate would prevent atomic oxygen from arriving at the unprimed canvas on this side. Exposing canvas directly to atomic oxygen for too long can cause removal of the sizing and weaken the canvas if not shielded from atomic oxygen arrival during cleaning. This outcome has been observed with some long exposures of unprimed canvas. Light cleaning of the stretcher and canvas on the back side could be achieved with a short exposure to atomic oxygen at the start of cleaning the primed and painted side. The cleaning would be achieved by moving the stretcher farther away from the ground plate so that there would be a gap of greater than 1 in. between the stretcher and plate.
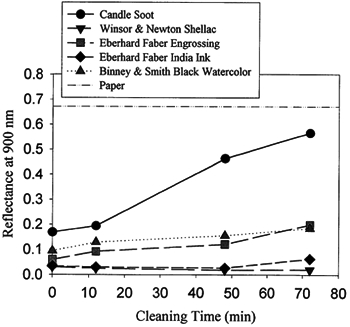
3.2 3.2 CLEANING OF ROY LICHTENSTEIN INK DRAWING ON PAPERThe Lichtenstein drawing proved to be a very difficult test subject for several reasons. Black ink typically contains a carbon pigment, so it was unknown whether the cleaning process could preferentially remove the soot without also removing the ink. Also, the paper appeared to have experienced thermal decomposition, as evidenced by a dark yellowing and browning and an increased brittleness. Therefore, it would probably be difficult to remove this damage from the paper without weakening it. Before attempting to clean the drawing, it was necessary to determine whether soot could be removed more quickly than ink. In order to test this possibility, 1.9 cm wide strips of various types of ink and watercolor, listed in figure 3, were applied to the surface of watercolor paper. A wide soot streak was also applied across the clean paper by passing it over the flame of a wax candle. The reflectance of light provided by a quartz halogen lamp, as described in section 2.3, was then monitored from each type of drawing medium and from the candle soot at various intervals during the atomic oxygen cleaning process. The resulting graph shown in figure 3 indicates that the candle soot is removed much more rapidly than the media tested, and the soot-coated paper is quickly brought back close to its original reflectance, as shown by the dashed and dotted reference line. All the tested media experienced some loss of material after about an hour of cleaning, except for the shellac-filled ink, which appeared to be very durable. This ink probably contains inorganic pigment that would greatly slow down the loss of the carbon in the ink due to reaction with atomic oxygen.
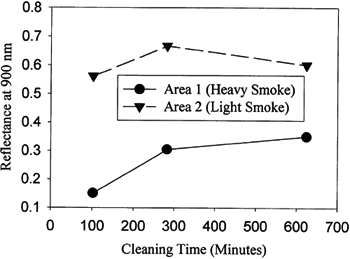
Each soot deposit from a fire will be of different composition according to the nature of the fire and the types of objects burning around the artwork. Therefore it is difficult to accurately determine the reaction rate for a specific drawing or painting. Some careful testing of the surrounding objects, such as a section of the frame, an edge of the painting, and other nearby objects, can give information about how long it will take to remove the soot deposit under the cleaning conditions that will be used. Eventually it is necessary to mask off all but a representative edge of the painting and expose this to atomic oxygen. This procedure will not only give an estimate for the cleaning time but also help to ensure that the pigments are safe for cleaning with atomic oxygen. Because the ink samples tested appeared to be a little more resistant to oxidation than first believed, it was decided to try first to clean an exposed corner of the Lichtenstein drawing, to determine if the paper could be lightened and if the type of media used for this drawing could be cleaned without being removed. The Lichtenstein drawing was masked with a sheet of polyimide Kapton roughly 0.005 cm thick by laying the Kapton over the drawing so that an edge of the drawing was exposed, and then taping the Kapton down to the matting around the drawing. Mylar or other polymer sheeting can also be used as long as it has been tested first to ensure that its reaction products will not transfer onto the painting surface as it oxidizes. After an initial cleaning of approximately 12 minutes, a visual inspection showed some lightening of the surface without affecting the ink areas. The mask was then removed, and the entire surface was cleaned for intervals of 12, 30, and 60 minutes. At this point, cleaning was stopped because it did not appear that the paper background could be lightened further without loss of some of the thinner ink features. At the request of the conservator, we masked off the bulk of the ink areas and tried to further lighten the paper in the upper left corner of the drawing. We again used a Kapton mask, but rolled up the edges of the mask to create a graduated cleaning that would prevent sharp cleaning lines from appearing on the surface. Figure 4 shows the reflected light data from two areas on the paper that were being cleaned, one with a light smoke residue and the other with a much heavier deposit. After a total cleaning exposure of about 300 minutes, there was no noticeable improvement in the drawing. The area still had a light yellow cast, which was likely due to thermal damage of the paper. Figure 5 shows photographs of the Lichtenstein drawing before and after cleaning with atomic oxygen.
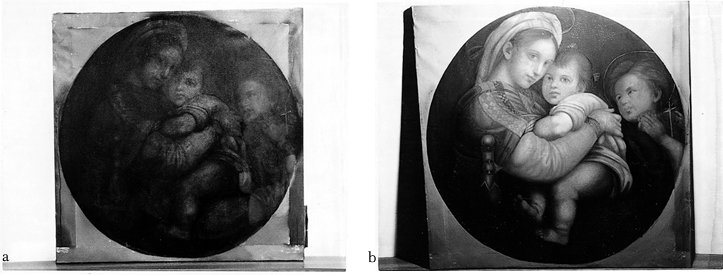
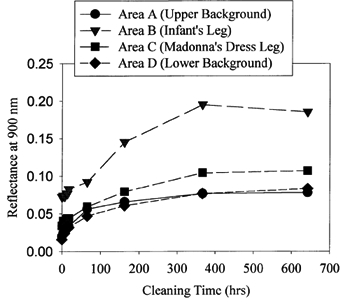
Some of the important lessons learned from the cleaning of this drawing were that areas could be successfully masked for cleaning without creating cleaning lines, and that ink and soot are removed at different rates. 3.3 3.3 CLEANING MADONNA OF THE CHAIRThe Madonna of the Chair painting posed a different type of challenge (see fig. 7a). This work had experienced very heavy smoke damage and some thermal decomposition. Areas of the varnish were charred in appearance. Prior to treatment, several areas were chosen on the painting for reflected light measurement at selected intervals during cleaning in order to determine the end point of the treatment process. The upper and lower background areas were selected because these should remain relatively dark and approximately the same during cleaning. The Madonna's garment was selected because it should clean to a higher level of reflectance than the background. The greatest contrast between the untreated and treated piece was expected to be the reflected light from the infant's leg. Cleaning would be considered complete when a change in the diffusely reflected light from these surfaces would no longer be measurable.
Figure 6 contains the reflected light data for the Madonna painting as a function of cleaning time. After approximately 350 hours, the majority of the darkened varnish was removed from the surface. There were still some thin streaks on the surface. The majority of these were removed after approximately 600 hours of treatment. Treatment was allowed to progress part of the way into the binder in order to remove the damaged portion as much as possible. This extent of cleaning was chosen for this painting because of the severe nature of the fire damage and the desire of the owners to bring the pigment back to the surface as much as possible. The process can be stopped earlier so as to minimize or prevent removal of the binder if desired. At the conclusion of the cleaning, the paint pigment was loosely bound on the surface. Because the atomic oxygen reaction is confined to the surface, it will remove binder that is in between and on top of pigment particles but will leave a small amount underneath that loosely attaches the particle to the surface. This result has been observed with early scanning electron microscope studies of oil paint exposed to atomic oxygen (Rutledge et al. 1994). Because the attachment point is small, it would be possible to remove pigment by mechanical contact with the surface such as brushing. Therefore, with the guidance of the conservator from the Cleveland Museum of Art, a fine spray mist of Grumbacher aerosol dammar varnish was applied to fix the pigment to the surface. A brush was then used to apply a thicker coating of Winsor & Newton dammar varnish over the surface. Figure 7 contains photographs of the Madonna painting as received (fig. 7a) and after cleaning with atomic oxygen and application of varnish (fig. 7b). The details of the painting are now clearly visible.
There is a potential for dehydration shrinkage during the treatment process because it is performed under a partial vacuum. For this painting, there was no deformation observed during treatment. It appeared that if dehydration shrinkage did occur, the canvas, paint, and stretcher shrank at approximately the same rate because no additional cracking beyond that originally present on the painting was observed after treatment. This result may not be the case for every material combination. A risk assessment should be made prior to treatment in this manner to determine if the shrinkage of the base material and the medium are closely matched or not. Paintings on some types of wood may be more at risk. This technique has typically been used to restore severely damaged objects, so the benefit of being able to salvage it has been much greater than the risk of shrinkage. Removal of all of the varnish on the surface and the top layer of binder material is not necessary in all cases. Some fire-damaged works, however, may require extreme cleaning to be restored so they can be enjoyed again. The authors were unable to obtain a photograph of the painting before the fire damage occurred, so it was difficult to determine if there were any shifts in paint coloration due to the treatment process. A number of oil paints have been tested for changes in coloration using reflectance spectroscopy before and after exposure to atomic oxygen (Rutledge et al. 1994). Changes in coloration were not observed for these materials, but they consisted of more modern paint formulations. Caution must be used when treating an untested paint medium using atomic oxygen if the cleaning will progress into the paint layer. A representative corner or edge of the painting that contains most of the paint colors present should be treated using atomic oxygen, with the remainder masked off, so that a determination can be made if this process will be safe for the pigments present. Organic pigments will experience oxidation and removal, so care must be taken during cleaning to minimize their loss. As more testing occurs, a greater knowledge base will be developed as to what types of paints and varnishes can or cannot be treated using this technique. With the proper precautions, atomic oxygen treatment does appear to be a technique with great potential for allowing previously unrestorable art to be salvaged. Further testing of this cleaning technique is being conducted to determine its effectiveness for cleaning smoke-damaged acrylic paintings and watercolors. 4 4. CONCLUSIONSAtomic oxygen treatment has been shown to effectively remove soot and charred varnish from a full-size oil painting and to be able to partially clean a fire-damaged ink drawing on paper. Masking techniques can be used to treat one area more extensively without leaving visible cleaning lines. Treatment can progress at the discretion of the conservator, from light surface-cleaning to more extensive removal of fire damage. In some cases, it may be necessary to remove some of the paint binder. This step would leave the pigment loosely bound on the surface. The pigment can be rebound by using a fine spray mist of a material of the conservator's choice. When cleaning, it is important to verify that the materials present in the artwork are safe for atomic oxygen cleaning by cleaning a small representative edge or corner prior to treatment of the entire painting. This technique appears to have great potential for removing soot and char from the surface of art damaged during a fire and may allow treatments of previously untreatable works of art. The process is not intended to be a replacement for conventional techniques, but as an additional conservation tool in applications where conventional techniques have not been effective. ACKNOWLEDGEMENTSThe authors would like to thank Mr. Richard Moore of Bonfoey Company in Cleveland, Ohio, and Mr. Kenneth B� of the Cleveland Museum of Art for donating some heavily fire-damaged paintings for our testing and also for providing very helpful comments, discussion, and advice. We would also like to thank Father Bob Weaver and the members of St. Alban's Episcopal Church for providing information about the Madonna painting and for their interest and enthusiasm surrounding its cleaning. REFERENCESBanks, B. A., S. K.Rutledge, J. A.Brady, and J. E.Merrow. 1988. Atomic oxygen effects on materials. In NASA/SDIO Space Environmental Effects on Materials Workshop. NASA conference publication 3035. Hampton, Va.: NASA. 197–239. Banks, B. A., and S. K.Rutledge. 1988. Low earth orbital atomic oxygen simulation for materials durability evaluation. In Proceedings of the Fourth European Symposium on Spacecraft Materials in the Space Environment, CERT, Toulouse, France, September 6–9. Toulouse, France: ONEAA Centre d'Etudes et de Recherches de Toulouse. 371–92. Banks, B. A., S. K.Rutledge, P. E.Paulsen, and T. J.Stueber. 1989. Simulation of low earth orbital atomic oxygen interaction with materials by means of an oxygen ion beam. Presented at the 18th Annual Symposium on Applied Vacuum Science and Technology. NASA TM-101971. Banks, B. A., and S. K.Rutledge. 1996. Process for non-contact removal of organic coatings from the surface of paintings. U.S. Patent # 5,560,781. Assigned to B. A. Banks and S. K. Rutledge. Dever, J. A.1991. Low earth orbital atomic oxygen and ultraviolet radiation effects on polymers. NASA TM-103711. Rutledge, S. K., B. A.Banks, and M.Cales. 1994. Atomic oxygen treatment for non-contact removal of organic protective coatings from painting surfaces. In Materials Issues in Art and Archaeology, vol. 4. Materials Research Society Symposium Proceedings 352, ed. P. Vandiver, J. Druzik, J. L. Galvan Madrid, I. Freestone, and G. Segan Wheeler. Pittsburgh: Materials Research Society. 161–66. NASA TM-106650. Rutledge, S. K., and B. A.Banks. 1996. Atomic oxygen treatment technique for removal of smoke damage from paintings. Presented at the Materials Research Society Meeting. NASA TM-107403. AUTHOR INFORMATIONSHARON K. RUTLEDGE has been employed by NASA Lewis Research Center for 17 years as a research engineer. Her primary focus as a member of the Electro-Physics Branch is on the durability and prevention of degradation of power system materials for satellites and spacecraft operating in low Earth orbit. She has a bachelor's degree in chemical engineering from Cleveland State University (CSU), and master's degrees both in materials science and engineering and in business from Case Western Reserve University in Cleveland, Ohio. Address: John H. Glenn Research Center, Lewis Field, Mail Code 309-2, 21000 Brookpark Rd., Cleveland, Ohio 44135-3191 BRUCE A. BANKS is the chief of the Electro-Physics Branch at NASA Lewis Research Center. He has been employed by NASA for 32 years as a physicist. His current primary field of interest is power system material durability in low Earth orbit. He has a bachelor degree in physics from Case Institute of Technology in Cleveland, and a master's degree in physics from the University of Missouri at Rolla. Address as for Rutledge. MARK FORKAPA was employed by NYMA Inc. as a mechanical design engineer. Currently he is employed by DuPont Tribon Composites in Cleveland, Ohio. He received a bachelor's degree in mechanical engineering from Cleveland State University. Address: DuPont Tribon Composites, 6200 Hillcrest Dr., Cleveland, Ohio 44125 THOMAS STUEBER provides electrical design services through NYMA Inc. He has a bachelor's degree in electrical engineering from Cleveland State University and is currently pursuing a doctoral degree. Address as for Rutledge. EDWARD SECHKAR provides mechanical design services through NYMA Inc. He has a bachelor's degree in civil engineering from Cleveland State University. Address as for Rutledge. KEVIN MALINOWSKI was an undergraduate student at Cleveland State University and performed research in mechanical design while working on-site at NASA for CSU. He recently graduated from the mechanical engineering program at CSU with a bachelor's degree. Address: Unavailable.
 Section Index Section Index |