DIFFERENCES IN IMAGE TONALITY PRODUCED BY DIFFERENT TONING PROTOCOLS FOR MATTE COLLODION PHOTOGRAPHSSYLVIE PENICHON
ABSTRACT—Matte collodion photographs were the dominant medium used by commercial portrait photographers at the turn of the century. These prints usually display a black neutral tone and have a matte surface and an excellent stability. For this reason, they have often been mistakenly identified as platinum prints. The tonality of matte collodion prints can also vary from purple to brown. This article will examine the possible factors responsible for these differences in tone. A brief history of printing processes will be given, followed by a description of the matte collodion process and the different toning protocols described in photographic treatises and journals of the period. Results of elemental analysis will also be discussed. TITRE—Diff�rences de tonalit�s produites par divers proc�d�s de virage pour les aristotypes au collodion mat. R�SUM�—Les aristotypes au collodion mat furent le support pr�f�r� des photographes portraitistes au tournant du si�cle. Ces tirages ont g�n�ralement un ton gris neutre, une surface mate et une excellente stabilit� chimique. Pour ces raisons, ils ont souvent �t� confondus avec des tirages au platine. La tonalit� des aristotypes au collodion mat peut varier du violet au marron. Cet article examinera les facteurs responsables de ces diff�rences de ton. Un bref historique, des proc�d�s de tirage sera pr�sent�, suivi d'une description du proc�d� au collodion mat et des diff�rents proc�d�s de virage d�crits dans les trait�s photographiques et les journaux de l'�poque. Les r�sultats d'analyses �l�mentaires seront aussi comment�s. TITULO—Las diferencias en la tonalidad de las impresiones fotogr�ficas al colodi�n mate como resultado de los m�todos originales de entonado. RESUMEN—El papel fotogr�fico al colodi�n mate fue el medio de impresi�n mas utilizado por los retratistas comerciales de fin de siglo. Estas im�genes se distinguen por su tonalidad neutra en negro, su superficie mate y su excelente estabilidad. Esto ultimo ha hecho que se identifiquen err�neamente como platinotipos. Por otro lado, el tono neutro de las fotograf�as al colodi�n mate puede variar desde el negro-viol�ceo hasta el color marr�n. Este articulo analiza los posibles factores a los que pueden atribuirse estas diferencias en tonalidad. Se dar� una rese�a hist�rica de los procesos fotogr�ficos de impresi�n, y una descripci�n del proceso al colodi�n mate y de los distintos m�todos de entonado que aparecen en revistas y tratados de fotograf�a de aquella �poca. Se expondr�n, por ultimo, los resultados obtenidos en los an�lisis de elementos. We believe permanency is the KEYSTONE of PHOTOGRAPHIC success, and all brands of paper' bearing our TRADE-MARK are manufactured on this principle. We hold our consumer's reputation and success identical with our own. We surround both with every safeguard known to Chemical science and our own experience.—Aristo Motto, American Aristotype Company, 1901. 1 INTRODUCTIONFor many years, caretakers of photograph collections and photograph conservators have mistakenly identified matte collodion prints as platinum prints. James Reilly (1986) finally acknowledged the wide use of this process over approximately three decades, from 1890 to 1920, in his book Care and Identification of 19th-Century Photographic Prints. It has since been 2 BRIEF HISTORY OF PRINTING PROCESSESThe first photographic printing paper, known as salted paper, was introduced in 1839 by William Henry Fox Talbot. It consisted of a sheet of paper soaked in a dilute solution of table salt and sensitized with a strong silver nitrate solution. The sensitized paper darkened rapidly when exposed to daylight, and the image appeared spontaneously without requiring chemical development. When exposure was judged sufficient, unexposed light-sensitive silver chlorides were eliminated in a fixing bath of sodium thiosulfate, or “hypo,” followed by a final rinse in water. Papers of this type are known as printing out papers (POPs) as opposed to developing-out papers (DOPs), which require chemical reduction of the silver salts to silver metal for the image to appear. The image of POPs is composed of photolytic silver, which literally means “separated by light.” Silver images occur in three basic structural forms: photolytic silver, physically developed silver, and filamentary silver. “Photolytic silver has the smallest particle size and produces red or brown image color. Size is directly proportional to the amount of light received during exposure” (Reilly 1986, 15). If not well processed or if exposed to an unfavorable environment, salted papers would fade and lose highlight detail over time. In 1850, the French photographer Louis D�sir� Blanquart-Evrard introduced a new printing material, the albumen paper that had a slightly glossy surface and provided images with much greater density range and contrast than the salted paper. Albumen paper was prepared by floating a sheet of paper on the surface of a beaten egg white solution containing ammonium chloride before sensitizing with silver nitrate. Later, the desire for more detail and greater sheen brought about double-coated paper with a heavy gloss. By the turn of the century, public taste had swung to the other extreme, and “matte” or even “rough” albumen papers were offered (Photo-Miniature 1907, 265). Albumen prints were processed in the same way as salted papers. The heretofore reddish brown hue of the image, which was considered inartistic and displeasing to the eye, could be altered to a more agreeable brown or dark purple by the use of a gold toner. Gold toning, or gilding, was introduced to photography in 1841 by Hippolyte Fizeau to improve the image contrast and stability of daguerreotypes (Fizeau 1841). The technique was adopted for paper prints in 1847 (Reilly 1986, 5). During the process of toning silver prints, some of the silver atoms are replaced with atoms of the toning metal that become distributed within the crystalline structure of the silver. The change in image color is a function of the alteration of particles' shape, size, and composition (Reilly 1986, 23). Toning also improves the image stability because silver alloys are much more resistant to oxidative-reductive deterioration than silver alone. This deterioration occurs when substances that oxidize the image silver (such as hydrogen peroxide, thiourea, and others) convert the metallic silver atoms to silver ions. The ions migrate away from the original site of the silver grains and are eventually reduced back to metallic, elemental silver. Albumen paper rapidly became the standard printing support used by photographers, but it was soon noticed that albumen prints could be unstable, showing a tendency to fade and discolor within a few years. The search for a new medium that would have the same desirable properties of albumen, without its instability, Carbon printing, introduced by Alphonse Poitevin in 1855, seemed to possess all the stability requirements. The light-sensitive media consist of a layer of dichromated gelatin containing a pigment. The dichromated colloid, selectively hardened by light through the negative, is then washed in warm water, where the unhardened areas dissolve away, leaving a positive image of pigmented gelatin. Carbon prints are very stable, and any color of pigment can be used. Although the process was improved successively by A. Fargier in 1860 and Joseph Swan in 1864, it was still delicate and costly, with many manipulations, and never became very popular for commercial portraiture (Nadeau The platinum print, or platinotype, came into use around 1880. The process was patented and introduced to the market by the Englishman Williams Willis in 1873 (Willis 1873). It consists of coating a sized sheet of paper with a light-sensitive solution of iron and platinum salts. After exposure to light, the paper is developed in a bath of potassium oxalate and then immersed in an acid bath that eliminates residual light-sensitive iron compounds from the paper. The final image is composed of metallic platinum. Platinum prints have a matte surface and a soft gray-black color, and are praised for their wide tonal range. However, inflation in the price of the metal imported from Russia starting in the 1880s drove the price so high that platinum paper cost about three times more than other papers (Aristo Eagle 1906). Platinotypes became too costly for general commercial use. The pre-eminence of albumen paper ultimately concluded with the arrival of emulsion papers, that is, papers precoated with a sensitized layer of collodion or gelatin binder containing suspensions of light-sensitive silver salts. These papers were industrially produced, which improved their quality and uniformity (fig. 1). They were also more sensitive than albumen paper and provided finer detail. Two types of light-sensitive salts were used in the emulsions: silver chloride and/or silver bromide. Collodion and gelatin chloride POPs became the dominant material from the late 1880s to the late 1910s. They were offered in a wide range of surfaces, image colors, and contrasts and did not share albumen's tendency to yellow (Reilly 1986, 10). Another type of silver chloride paper, known as “gaslight” or development paper, was introduced to the market by the Nepera Chemical Co. in 1893, under the name of Velox. The paper, coated with a gelatin silver chloride emulsion, was sensitive enough to be printed under artificial light (gaslight). The image was developed chemically (Nadeau 1989, 114).
Gelatin silver bromide paper was introduced at the same time as bromide dry-plate negative. More sensitive than chloride papers, it could be printed with a very short exposure to daylight or with any artificial light. The image was brought out through development. Bromide paper was essentially used for enlargements. Although it was commercially available in the mid-1880s, it did not become popular until much later. Commercial photographers were used to controlling the exposure time visually, as the image was appearing to the printing frame, and to working the paper throughout in daylight. They found the trial-and-error method for bromide DOPs cost them time and money; the paper was more expensive and did not produce the wide range of tones that were possible with chloride POPs. However, bromide paper eventually became the dominant printing material and is still in use today. 3 COLLODIO-CHLORIDE PAPERSThe first use of collodion as a binder for paper prints can be attributed to Antoine Gaudin, who described his method of preparing collodion photog�ne—a name he applied to “any sensitive compound containing nitrate of silver”—in the pages of La Lumi�re of September 1861 (quoted in Harrison 1887). Collodion was a substance already known by the photographic community, for it was used as a binder in processes such as the ambrotype, the tintype, or the wet collodion negative. In these processes, a solution of collodion containing potassium iodide was poured over a metallic or glass support and immediately dipped in a sensitizing bath of silver nitrate. The difference in Gaudin's experiment was that the sensitizing bath was dispensed with by the inclusion of light-sensitive silver salts within the collodion binder, and the emulsion was ready for use at once. On March 17, 1865, George Wharton Simpson, editor of Photographic News, presented a paper entitled “On a New Method of Printing and the Preparation and Use of Collodio-Chloride of Silver,” at a meeting of the London Photographic Society. In his presentation, Simpson stressed the difficulties of obtaining consistent results with the floating method currently used with albumen papers, because every sheet floated over a sensitizing solution changed the composition of this solution. With one batch of emulsion this problem would disappear. The first sheet of paper coated had the same properties as the last one. He explained:
Simpson experimented with a wide variety of papers, including “Whatman's drawing paper, Turner's Calotype paper, Bristol-board, common writing paper, ordinary Saxe and Rive paper, and the paper prepared with arrowroot for the Wothlytype process” (Simpson 1865, 122), which he coated with his collodion emulsion. The best samples he presented at the meeting were made on paper sized with arrowroot starch, which provided a better adhesion of the emulsion to the paper and gave the surface an evenness not achieved with unsized papers. The prints aroused great admiration, but the process was not disseminated until several years later. Around 1866, the Soci�t� Leptographique introduced in France the Leptographic paper, invented by two photographers in Madrid, Jos� Martinez-Sanchez and Jean Laurent (Werge [1890] 1973). The paper was a modification of Simpson's collodio-chloride recipe and one of the first printing-out papers to make use of barium sulfate in its manufacture By the end of the 1870s, the commercialization of ready-to-use gelatin dry-plate negatives provoked a rapid expansion of the market. The growing number of amateur photographers instigated an enormous demand for ready-made photographic papers (fig. 2). This demand induced the improvement and mass production of both collodion and gelatin POPs (Wentzel 1960). In 1884, Paul Eduard Liesegang of Dusseldorf started the manufacture of a fine collodion emulsion printing paper called Aristotype, from the Greek aristos (best) and tupos (type). A formula of POP using gelatin was published in 1882 by W. Abney, an English photochemist. Gelatino-chloride paper was introduced in England by Ilford Ltd., in 1885, and soon after in other countries (Sturge 1977, 5). Machine production of collodion POPs on continuous rolls did not start until 1889, when Dr. A. Kurtz introduced his glossy Celloidin paper in Germany (Eder [1932] 1972; Nadeau 1989, 61). Technology for the production of collodio-chloride POP in the United States was introduced by Carl Christensen. After the American Aristotype Company was founded in 1889, other smaller companies followed, including Kuhn Crystallograph of Springfield, Missouri; Western Collodion Paper of Cedar Rapids, Iowa; Bradfisch Aristotype of Brooklyn, New York; United States Aristotype and New Jersey Aristotype, both of Bloomfield, New Jersey (Jenkins 1975, 87). Production of gelatin-chloride papers followed in the early 1890s. Both papers were initially produced with a glossy finish that appealed to the taste of the 1880s. American Aristo and its later varieties, Aristo-Platino, Aristo-Carbon, Aristo-Junior, etc., all collodio-chloride papers, were
Self-toning collodion papers were first introduced in Germany by W. M. Ashman and B. Offord in 1885 (Wentzel 1960, 69) and became very popular with amateurs because they were fixed and toned in the same bath without any washing or other preliminary treatment (fig. 3). The basis of the emulsion for these papers was “fulminating gold [gold fulminate], together with chloride and silver nitrate, citric acid, and glycerin dissolved in collodion” (Salmon 1921). The gold salt contained in the emulsion was reduced in the fixing bath, which toned the image, preventing the need for a separate bath. One great merit of self-toning papers was the evenness of tone available throughout a series of prints (Photo-Miniature 1910). Some self-toning papers were also prepared with salts of selenium or tellurium (Clerc 1937, 356).
The diffusion of ready-made photographic paper was rapid within the amateur market, but it progressed more slowly among the professionals, who were reluctant to adopt it. Although collodio- and gelatin-chloride papers had many advantages over the albumen process (including ease of use, evenness of coating, more stability, increased sharpness of details in the shadows), years of practice with the latter led to its domination of the marketplace. With the new paper, everything had to be started over. Numerous articles and letters published in photographic journals attest to the suspicion photographers had toward machine-made emulsion paper, as illustrated by these comments:
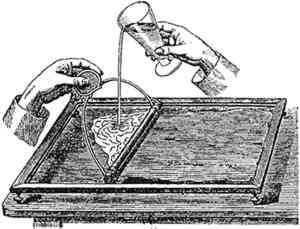
Wide adoption of sensitized papers by commercial photographers did not come about until the early 1890s (Jenkins 1975, 81). At the same time, the public developed a taste for the matte papers and neutral black tones that were characteristic of the platinum print. This process had gained the favor of artists such as Clarence White, Frank Eugene, and Henrich Kuehn, but the prohibitive price of the metal was incompatible with commercial practice. The process was also judged to be lacking in sharpness and contrast for commercial portraiture. Matte collodion papers that had the platinotype's matte surface and neutral image color were introduced around 1894 (Nadeau 1989, 71) to answer both aesthetic and economic needs. By the end of the decade, their use was predominantly for portraits. In 1907, we learn that “probably 75% of the printing paper used by American professional photographers is Aristo-Platino,” a matte collodio-chloride paper (Photo-Miniature 1907). The situation lasted until about 1910, when printing-out papers were supplanted by faster gelatin developing-out papers (Reilly 1986, 12). (See fig. 4.)
4 STRUCTURE OF PRINTING-OUT PAPERSIn printing-out papers, the sensitive material is silver chloride associated with an excess of soluble silver salts capable of absorbing halogen liberated by the action of light. The fast darkening of POPs is a consequence of this excess of soluble silver salts contained in the support itself, in the binder, or in the emulsion. The image becomes visible upon exposure to light, without the action of a developer. It consists of metallic silver in a very fine state of division (photolytic silver). The tone of the image depends on the size, distribution, and morphology of the silver particles (smaller particles absorb the smallest wavelengths and display a warm, reddish tone; bigger particles tend to give prints of cooler tone). Unlike albumen papers, where the silver particles are small and concentrated near the surface of the binder, emulsion papers have large silver particles that are spread uniformly in the binder (Lav�drine 1990). 5 PREPARATION OF THE EMULSIONCollodion is prepared by dissolving a cellulose nitrate compound in a mixture of alcohol and ether. The proportions of ether and alcohol may vary according to the operator or manufacturer. If too much alcohol is added, the film becomes streaky; if ether is in excess, the film becomes too contractile and liable to split when drying. Aged collodion, rather than fresh, was preferred for the making of emulsions: “Collodion, like wine, improves by keeping,” wrote W. E. Woodbury ([1898] 1979, 111) in his encyclopedia. The emulsion was sensitized by mixing soluble silver salts (silver nitrate) into collodion containing an organic acid (citric or tartaric) and a chloride (strontium, lithium, calcium, etc.) so that silver chloride and other salts (strontium nitrate, silver citrate, etc.) were formed by precipitation (Clerc 1937, 349). A few drops of castor oil were often added to the emulsion to give suppleness and avoid risk of cracking (Clerc 1937, 349). It was also believed that the oil would assist the toning and be 6 COATING OF THE PAPERBefore the industrial manufacture of collodiochloride paper, the operator himself had to prepare his paper (fig. 5). One could buy paper already coated with barium sulfate mixed with gelatin (baryta) at photographic suppliers. The method most frequently used by photographers to coat their paper was a technique directly inherited from the wet-plate process: the sheet of paper to be coated was secured to a rigid support and the light-sensitive emulsion was poured in the middle of it, as in the coating of wet-plate negatives. The paper was then tilted gently until its whole surface was coated, never allowing the emulsion to flow twice on the same portion of the paper. A thin film was preferred to a thick one because it gave a more even coating (Woodbury [1898] 1979, 113).
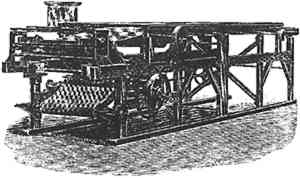
A. Kurtz introduced the first coating machine in 1889. Long rolls of baryta paper were pulled over a container of emulsion and brought quickly to a level position. Then the paper ran through a long tunnel box where a current of warm air blew (fig. 6). The paper was set and dry when it reached the end of the tunnel where it was cut up into sheets (Woodbury [1898] 1979, 114). The sheets were stored face to face with an interleaving of straw paper to regulate moisture (Wentzel 1960, 70). The paper had a shelf life of a few months if kept from light and excessive humidity.
In June 1893, an editor of Photographic Times visited the New York Aristotype Company in Bloomfield, New Jersey, and gave a brief account of the production of gelatin and collodio-chloride paper:
One advantage of producing collodioncoated emulsion paper over gelatin was that production could take place in a smaller room. The collodion paper dried very rapidly due to the fast evaporation of ether, and it did not need to be hung up in festoons like gelatin (Woodbury [1898] 1979, 113). Collodion emulsion was also easier to make than gelatin, because it could be done at room temperature, though it was rather more difficult to coat (Baker 1904). However, the tendency of the paper, when coated, to curl up at the sides was a problem, and special devices had to be installed on the coating machines to prevent the paper from curling up (Woodbury [1898] 1979, 113). Furthermore, guncotton could easily explode, and solvent fumes were very hazardous. 7 PROCESSING, MOUNTING, AND FINISHINGThe traditional sequence of baths for processing gelatin or collodio-chloride papers after exposure in the printing frame was: washing, toning, fixing, and washing, although toning and fixing would sometimes be done in one combined bath. A clear water bath was required before toning to eliminate the excess of soluble silver salts in the emulsion. To compensate for the weakening of image with the dissolution of silver salts that occurred during washing, the print had to be overprinted. Because prints dry up “darker and bluer” (Wallace 1888, 164) than when seen in the toning bath, the correct tone had to be checked by viewing the print with transmitted light during processing. A hardening bath made of chromium alum and formaldehyde was sometimes used between toning and fixing. It was to prevent excessive swelling and softening of the gelatin contained in the baryta layer that would result in the collodion film peeling right off the paper (Baker 1904). The collodion prints were usually mounted when still damp. Spotting was done with either gelatin, gum, or albumen mixed with some pigment (Woodbury [1898] 1979, 116). If a glossy surface was desired, the print was burnished or squeegeed face down against a sheet of glass and allowed to dry in this position. Before mass production of matte collodion papers, matte surfaces were obtained with the same technique on ground glass. Woodbury describes another alternative:
Later, a matte surface was obtained by using a coarse layer of baryta under the emulsion (Bentzen 1926). Rice starch was sometimes added to the baryta to achieve a matte surface (Wentzel 1960). 8 PROS AND CONS OF COLLODION PAPERAlthough it was almost impossible to identify a collodion from a gelatin print once it was mounted and presented to the patrons, some difference existed in the processing. For example, gelatin paper was not as well suited for producing the wide range of tones readily obtained with collodion paper. In June 1897, Wilson's Photographic Journal recommended the use of collodion paper to its readers because it possessed many advantages over gelatin:
During the summer or in warm regions, collodion paper was preferred over gelatin because its emulsion did not swell and was more resistant to water. Gelatin paper also had to be washed longer than collodion paper because the various processing solutions penetrated more thoroughly in the swelled emulsion. Collodion paper did have the disadvantage of curling in the baths. To avoid this problem, photographers would sometimes coat the back of the paper with an alcoholic varnish or with plain collodion, but this method was considered too costly for commercial practice. An alternative was to lay the prints face down in a tray filled with warm water and let them sit there for about 10 minutes, while gently patting them (Woodbury [1898] 1979, 115). Collodion prints were also more susceptible to chemical impurities such as sodium thiosulfate (fixer) residue in the blotters in which they were left to dry and to finger prints (Photo-Miniature 1910). 9 THE COLOR OF SILVER CHLORIDE PRINTSAs stated above, a wide variety of tones could be obtained with collodio-chloride papers (fig. 7). During his presentation to the London Photographic Society in 1865, Simpson said:
According to the literature on silver chloride
This comment explains in part why photographers were reluctant to adopt the new emulsion papers in their commercial practice. Detailed descriptions of the effects of each of these factors follow. 9.1 CONDITIONS OF EXPOSUREMany authors noted that exposing the negative in direct sunlight gave images of lesser contrast: “Printing in the shade will give the pluckiest results, while strong sunlight is apt to give soft prints,” wrote T. T. Baker (1904, 483). If the printing was carried out in intense light, the darkening was quick and the midtones could be destroyed. The tone of the resulting picture was red (Hasluck 1906, 240). When the negative was exposed to well-diffused light, the tones were purple. P. N. Hasluck (1906) proposed that the difference in tone was caused by a more gradual reduction of the silver. According to L. P. Clerc (1937, 346), the degree of humidity of the emulsion considerably affected the color of the image: “A damp condition increases the sensitivity of the organic salts [citrate of silver for example] without affecting that of the chloride, and so, for a given exposure, the image is redder than that given by a dry film.” 9.2 NEGATIVE QUALITY“The first factor of tone in the print is the character of the negative,” wrote Wallace (1888, 163). Strong and contrasty negatives gave purple 9.3 EMULSION FORMULAThe type of acid and chloride selected for the making of the emulsion influenced the final tone of the print. The final tone also depended on the alcohol, ether, and water content of the collodion. For example, a higher alcohol content would give a more reddish tone, while higher proportions of water and ether would provide a bluer tone (Wentzel 1960, 70). Table 1 recapitulates the possible tone variations directly related to the emulsion's formula. TABLE 1. TONE VARIATIONS RELATED TO EMULSION'S FORMULAE The image's overall tone depended not only on the composition of the final image material but also on the color of the highlights. To achieve desired effects, baryta, emulsion, and/or paper support were sometimes tinted. The addition of a red dye (cochineal, for instance) to the baryta layer resulted in a warmer tone, especially desirable for portraits, while a blue dye gave pictures a colder appearance, more appropriate for landscape (Wentzel 1960). “Dyestuffs such as methylviolet [a dye consisting essentially of pentamethyl-pararosaniline chloride] are added in very small amounts [into the emulsion] to cover a slight tendency to yellowing of the paper caused sometimes by the collodion or the tint of the baryta layer” (Wentzel 1960, 70). 9.4 METALS USED FOR TONING BATHToning was a standard procedure in the processing of POPs. A. Davanne and A. Girard gave in 1864 the following definition for toning:
Early investigators used the word “coloring” rather than “toning” to describe the process of changing the image tone (Lee 1987). While the literature offers a wide variety of recipes, gold toners, platinum toners, or a combination of both metals were most commonly used for silver chloride prints. At first, gold toners that gave nearly black tones were recommended. Soon, however, a combination of gold toning followed by platinum toning became standard for the processing of matte collodion printing-out papers (Reilly 1986, 12). Table 2 summarizes the most common toning baths used for printing-out papers and the tone they would provide. TABLE 2. COMMON TONING BATHS USED FOR PRINTING-OUT PAPERS Gold toner was the most widely used toning bath. A wide range of tonalities, from sepia to purplish black, could be obtained with a gold toning bath. The action of gold chloride on the silver image can be expressed by the following equation: Ag + Au Cl → Ag Cl + Au. The size and, thereafter, the color of finely divided gold depends very much upon the rate at which gold is deposited, that is, the speed of toning. This rate depends on the alkalinity of the bath. In an alkaline medium, gold (III) chloride (AuCl3) changes progressively into gold (I) chloride (AuCl), which deposits three times as much gold per unit amount of silver as the auric salt (Clerc 1937, 350). However, if the bath is too alkaline, the gold (I) salts change to the inactive aurite state, and the toning does not take place (Clerc 1937, 350). Neutral and slightly alkaline baths tone very quickly because the reduction takes place rapidly. The gold particles appear blue because they are somewhat large. In an acidic bath, reduction takes place very slowly. The gold is deposited in a dispersed form and reddish images are produced because of the extremely fine state of division of the gold. Clerc (1937, 350) noted that alkaline gold toning baths that were recommended for salted and albumen papers could be used for gelatin printing-out papers but that they were “almost without action on collodion papers; these latter should be toned in a bath containing a solvent of silver chloride, e.g. sulfocyanide or thiourea, which can slowly make its way within the collodion.” The use of platinum as a toning agent was first proposed by Ernest de Caranza (1856) who added hydrochloric acid to his toner, but the acid caused the bleaching of the print. A practical method of platinum toning was proposed in 1890 by Lyonel Clark, who solved this problem by using nitric acid (Burbank [1891] 1973). The action of platinum salt can be expressed by the following equation: Ag + KPtCl2 → KCl + AgCl + Pt. Platinum toners gave collodio-chloride papers warm brown to sepia tones. They were seldom used for glossy varieties of papers, and thought to be more suitable for matte surface papers (Hasluck 1906). Gold toning followed by a platinum toning bath gives a black neutral tone. The platinum tone was to a great extent controlled by the gold toning: the shorter the toning in gold, the warmer the final tone (Photo-Miniature 1910). This writer does not know when palladium toning was adopted. A. Reynolds suggested the use of palladium as a printing medium around 1890, and palladium paper was commercially introduced as a cheaper substitute to platinum around 1916 (Nadeau 1989, 355). It seems reasonable to assume that palladium toners were introduced at the same time, for the same reasons. Gold and palladium toning gave approximately the same tone as gold and platinum. Selenium toning was introduced in 1910 (Lee 1987) and replaced the expensive platinum and gold during World War I. Toning was done after fixing to avoid staining of the whites. Gold and selenium gave the same tone as platinum if the photograph was fixed prior to its immersion in the selenium toning bath. Lead was used in combination with gold in combined fixing-toning baths. It gave purple tones. A combined toning and fixing bath was first suggested in 1850 by Gustave Le Gray, but it was not employed until about 1890 (Clerc 1937, 354). If not used properly, this technique could produce very unstable prints due to incomplete fixing or exhausted bath. Baker (1904, 492) noted that “combined toning and fixing is more applicable to collodion than to gelatin printing-out papers.” 9.5 DURATION, TEMPERATURE, AND CONCENTRATION OF TONING BATHMost authors agree that different results can be obtained from the same formula if different working methods are employed. The quantity of metal salts in the toning bath also influenced the final color of the print. A greater proportion of gold, for example, gave a bluer and colder tone, while little gold in the bath gave from reddish to warm brown tones (Hasluck 1906, 245).2 The shorter the time in the gold toning bath, the warmer or redder the color of the finished print. The longer the print remained in the toning bath, the bluer or colder it became (Bothamley n/d, 102). Overtoning gave the print a cold and faded appearance (Burbank [1891] 1973, 40). If the bath was too cold, the tendency was to get a red print because gold was formed slowly (Hasluck 1906, 245). The recommended proper temperature of the bath was usually set around 60� F. 10 ENERGY DISPERSIVE X-RAY SPECTROMETRY (EDS) ELEMENTAL ANALYSISThe versatility of the matte collodion process and the infinite variety of tones it provided were repeatedly praised in the literature of the period. The second part of this study consisted of the elemental analysis of several matte collodion photographs in order to establish a relationship between toner and final color of the print. Energy dispersive x-ray spectrometry (EDS) is a nondestructive analytical technique for determining the elemental composition of a material. The technique itself is non-destructive, but it is often thought of as destructive because the analysis is usually done on samples taken from objects rather than on the object itself. Other nondestructive methods, such as x-ray fluorescence (XRF), where samples are not placed in vacuum during the operation and prints of virtually any size can be analyzed, are usually preferred by conservators for elemental analysis. There are two main advantages of EDS microanalysis over XRF for this project. First, EDS is usually done at a lower voltage The EDS detector measures the energies of the characteristic x-rays produced by each element in the material. These x-rays are produced after bombardment of the sample with a beam of electrons, usually from a scanning electron microscope (SEM). By measuring the energies of the different x-rays produced, EDS can qualitatively identify which elements are present in a material. By counting the number of each energy x-ray produced, EDS can also provide a quantitative analysis of the composition. Eight matte collodion photographs (fig. 8) were selected from study collections so the widest variety of image tonalities would be represented. Because matte collodion prints are sometimes difficult to distinguish from matte gelatin prints through visual examination only, a spot test was applied on every photograph to confirm that the binder was collodion. The test consists of applying a drop of deionized water on the photograph and letting it sit for a minute before it is dried with a blotter. The wet area is then observed under a microscope. Unlike gelatin that swells when it comes in contact with water, collodion does not absorb water and will not show any change. Sampling and analyses were performed by Mark Wypyski, associate research scientist at the Sherman Fairchild Center for Objects Conservation of the Metropolitan Museum of Art, New York. The photographs were sampled in the darkest areas of the image, where more image components (silver and toning metal) are expected to be found. Samples measuring approximately 0.25 square mm were taken using a steel scalpel and mounted onto carbon sample mounts with conductive carbon paint. The analyses were done using a Kevex model Delta IV EDS attached to
TABLE 3. ELEMENTAL ANALYSIS OF MATTE COLLODION PHOTOGRAPHS All the samples showed large amounts of sulfur and barium, ranging from 55 to 77% of the sample's total composition. This finding indicates that a great amount of baryta (barium sulfate and gelatin) layer was taken together with the binder during the sampling of the photograph. Aluminum and silicon were present in variable amounts in all the samples. These elements constitute “background noise” to any material being analyzed; their presence can be discounted. In addition to the silver image, three of the images analyzed revealed the presence of gold only; four showed a combination of gold and platinum; one had platinum only. Print 1, showing three adults, is a very slightly gold-toned photograph. Gold represents only 2% of the total composition of the sample and 8% of the image material. The tone of the print is brownish red and resembles the color of an untoned POP. This color is not often seen in matte collodion prints. The sample taken from Print 2, showing a standing woman with a hat, contains a greater percentage of gold than silver. The photograph displays a reddish purple tone. Print 3, showing a seated man, was gold toned to a dark purplish color. The large amount of gold in the sample, 65% of the image material, indicates a thorough substitution of the silver image. Print 4, showing a girl with a ribbon in her hair, is a gold-platinum-toned photograph. There is much more gold than platinum in the sample and the photograph displays black neutral tone. Silver remains the main component of the image material indicating short gold and platinum baths. The same observation can be made for Print 5, a gold-platinum-toned photograph of Print 6, showing a seated girl, is a gold-platinum-toned photograph. This time, gold and platinum are in equal amounts in the sample. The color of the print is a neutral, somewhat washed gray. Print 7, showing three children, is a gold-platinum-toned photograph that displays a neutral black hue. The amount of platinum in the sample is twice the amount of gold. Print 8, showing two young women, was toned with platinum only and displays a brownish gray tone. 11 CONCLUSIONSThe arrival of commercial, ready-to-use photographic papers was a benchmark in the history of photography. It completed the movement that had been initiated with the introduction of the dry-plate negative, and photographers progressively lost control of the production of photosensitive materials. Matte collodion photographs were popular from approximately 1894 to 1920 and were introduced to supply an aesthetic need for matte surfaces and neutral tones. Although the literature offers a wide variety of toning recipes, it is often mentioned that gold, platinum, or a combination of both toners were the most commonly used for matte collodion prints. The limited number of photographs analyzed in this study does not authorize a conclusion about the predominant use of gold or combined gold and platinum toners; however, the results do agree with this statement. This study also revealed that photographers praised collodio-chloride papers mainly because any color, from red-brown to a deep rich purple-black, or even a cold black, could be obtained with them. The factors responsible for these color differences such as the conditions of exposure, the negative quality, the emulsion formula and paper brand, the metal used for the toning bath, the toner concentration, the time in the bath, and the temperature of the bath, were identified through the literature of the period and the study of the mechanism of toning. Analysis confirmed that a toning bath could produce prints of varied tones. Gold toning, for example, can provide almost neutral tones if the substitution of photolytic silver for gold-silver alloy is thorough. A trained eye might be able to distinguish correctly different toning protocols (gold versus gold/platinum for example), but positive identification of the toner is almost impossible without elemental analysis. ACKNOWLEDGEMENTSThe author would like to thank Mark T. Wypyski and the Metropolitan Museum of Art for the analysis; Nora Kennedy, Doug Nishimura, and Doug Munson for their input during the project; and Peter Mustardo, Debbie Norris, and James Reilly for the samples of matte collodion photographs. Thanks also go to Peggy Ellis, Doug Severson, and Carol Turchan for their revisions and suggestions. NOTES1. Chloride of gold = gold (III) chloride; sulphocyanide of ammonia = ammonium thiocyanate; sel d'or = gold chloride used in combination with sodium thiosulfate (see table 2, combined toning/fixing); hyposulfite of soda = sodium thiosulfate. 2. Hasluck (1906) gives the following proportions of gold for different tones: Mass equivalent (grain) was derived based on the assumption of a 1% solution. There are approximately 17 British minims per ml, and REFERENCESAristoEagle. 1901. Aristo motto. Aristo Eagle1(2): 1. AristoEagle. 1905. Aristo Eagle. 5(1): 16–17. AristoEagle. 1905. Aristo self-toning ad. Aristo Eagle5(11): 26. AristoEagle. 1906. Platinum goes up again. Aristo Eagle6(8): 20. AristoEagle. 1908. Aristo self-toning ad. Aristo Eagle7(4): 22. Baker, T. T.1904. Printing-out papers. Photo-Miniature6(6):469–99. Bentzen, T.1926. Emulsions and baryta coating. British Journal of Photography73 (January):59–62, 73 (August):499–500. Bothamley, C. H. n.d. The Ilford manual of photography. London: Ilford, Ltd. Burbank, W. H. [1891] 1973. Photographic printing methods. Reprint, New York: Arno Press. Clerc, L. P.1937. Photography, theory, and practice. 2d ed.. New York: Pitman Publishing Co. deCaranza, E.1856. Nouveau proc�d� de fixage des �preuves positives au moyen du chlorure de platine. La Lumi�re6(8):29. Eder, M. J. [1932] 1972. History of photography, trans. Edward Epstean. Reprint, New York: Dover Publications. Fabre, C.1890. Trait� encyclop�dique de photographie. 2 vols. Paris: Gauthier-Villars. Fizeau, H.1841. Sur un moyen de fixer les images photographiques. Comptes rendus hebdomadaires des s�ances de l'Acad�mie des sciencesII:237–38. Harrison, J. W.1887. Chapters in the history of photography. Collodion emulsion. Photographic Times and American Photographer17 (June):225. Harrison, J. W.1892. The toning of photographs considered chemically, historically and generally. In The chemistry of photography. New York: Scovill and Adams Co.334–359. Hasluck, P. N., ed.1906. The book of photography, practical, theoretic and applied. London: Cassel & Co. Jenkins, R. V.1975. Images and enterprise: Technology and the American photographic industry, 1839 to 1925. Baltimore and London: Johns Hopkins University Press. Lav�drine, B.1990. Les Aristotypes, pt. 1. Preprints, ICOM Committee for Conservation, 9th Triennial Meeting, Dresden. Paris: ICOM. 235–61. Lav�drine, B.1991. Les Aristotypes. Les documents graphiques et photographiques: Analyse et conservation. In Travaux du CRCDG 1988–1989. Paris: Archives nationales, La Documentation fran�aise. 149–219. Lee, W. E.1987. Toning: Its invention and expanding role in photography. In Pioneers of photography, ed.E.Ostroff. Springfield, Va.: Society for Imaging Science and Technology. 72–78. Legros, V.1891. L'Aristotypie. Paris: Soci�t� d'�ditions scientifiques, Biblioth�que g�n�rale de photographie. Nadeau, L.1982. History and practice of carbon process. Fredericton, Canada: Atelier Luis Nadeau.
Nadeau, L.1986. History and practice of platinum printing. Fredericton, Canada: Atelier Luis Nadeau. Nadeau, L.1989. Encyclopedia of printing, photographic, and photomechanical processes. 2 vols. Fredericton, Canada: Atelier Luis Nadeau. Nishimura, D.1998. Personal Communication. Image Permanence Institute, Rochester, N.Y. Photo-Miniature. 1907. Printing processes described. Photo-Miniature7 (78):263–71. Photo-Miniature. 1910. Six photographic printing processes. Photo-Miniature9 (108):531–75. Reilly, J. M.1986. Care and identification of 19th-century photographic prints. Rochester, N.Y.: Eastman Kodak Co. Salmon, P. R.1921. The modern history and manipulation of self-toning paper. British Journal of Photography68 (December 9):730. Simpson, G. W.1865. On a new method of printing and the preparation and use of collodio-chloride of silver. Paper presented at a meeting of the London Photographic Society March 14. Photographic News9 (341):121–23. Spiller, J.1870. The collodio-chloride process. The Photographic News14 (March 25):136–37. Sturge, J. M.1977. Neblette's handbook of photography and reprography. 7th ed.New York: Van Nostrand. The Amateur Photographer. 1925. August 12, 30. Wallace, E.1888. Printing methods. American Journal of Photography9 (7):163–65. Welling, W. B.1978. Photography in America: The formative years, 1839–1890—A documentary history. New York: Thomas Y. Crowell Co. Wentzel, F.1960. Memoirs of a photochemist. Ed.Louis WaltonSipley. Philadelphia, Pa.: American Museum of Photography. Werge, J. [1890] 1973. The evolution of photography. Reprint, New York: Arno Press. Willis, W.1873. Improvements in photo-chemical printing. British Patent #2011, June 5, 1873. Reprinted in Nadeau 1986. Woodbury, W. E.1893. Aristotypes and how to make them. New York: Scovill & Adams Co. Woodbury, W. E. [1898] 1979. The encyclop�dic dictionary of photography. New York: Scovill & Adams Co. Reprint, New York: Arno Press. Wypyski, M.1998. Personal communication. The Metropolitan Museum of Art, New York. FURTHER READINGBaker, T. T.1948. Photographic emulsion technique. Boston: American Photographic Publishing Co. Burbank, W. H.1891. Platinum toning. American Amateur Photographer3 (7):249–52. Crawford, W.1970. The keepers of light: A history and working guide to early photographic processes. Dobbs Ferry, N.Y.: Morgan & Morgan. Formstecher, F.1921. The production of various colours on gold toned prints. Photographische Rundschau57:162–64. Hanneke, P.1897. Das celloidinpapier, Seine Herstellung und Verarbeitung. Berlin: Verlag von Gustav Schmidt. Morgan, W. D.ed.1949. Encyclopedia of photography. New York: National Educational Alliance. Neblette, C. B.1927. Photography: Its principles and practice. New York: D. Van Nostrand. Reilly, J. M.1980. Albumen and salted paper book. Rochester, N.Y.: Light Impressions.
Wall, E. J.1929. Photographic emulsions. Boston: American Photographic Publishing Co. Wall, E. J.1940. Photographic facts and formulas. 2d ed.F. I.Jordan. Boston: American Photographic Publishing Co. AUTHOR INFORMATIONSYLVIE PENICHON graduated cum laude from the Federal University of Rio de Janeiro, Brazil, with a B.A. in communications. She received a certificate of training in photographic preservation and archival practice from the George Eastman House, Rochester, New York. She received her M.A. in art history and diploma in conservation from the Institute of Fine Arts, New York University, specializing in the conservation of photographs. She is currently an Andrew W. Mellon Fellow in photograph conservation at the Art Institute of Chicago.
 Section Index Section Index |