THE DEVELOPMENT AND INITIAL APPLICATION OF A GAS CHROMATOGRAPHIC METHOD FOR THE CHARACTERIZATION OF GUM MEDIASARAH L. VALLANCE, B.W. SINGER, S. M. HITCHEN, & J. H. TOWNSEND
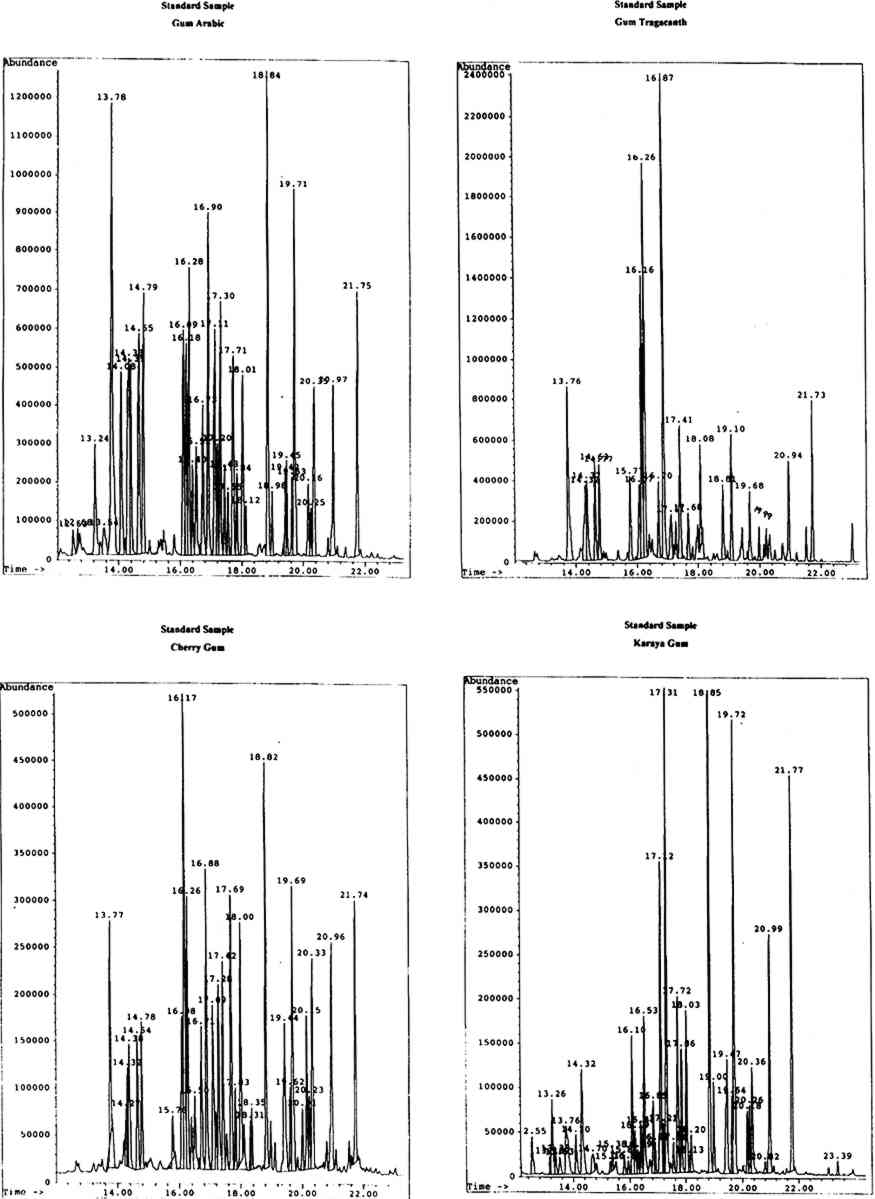
4 RESULTS AND DISCUSSIONThe identity of gums used as artists' media was ascertained by the analysis and identification of the monosaccharide components of the materials. Chromatographic data were first obtained for the standard monosaccharide samples, for comparison with that of the standard gum media. The chromatograms of the artificially aged and unaged standard gum media were very similar; hence the chromatograms obtained for the artificially aged gums were chosen to assist with identification of samples. 4.1 STANDARD GUM MEDIAResults for the individual standard monosaccharides will be considered first. A number of peaks were seen for each sugar, to a greater or lesser degree, corresponding to different structural forms of the silylated derivatives. table 1 shows the retention data for the individual monosaccharides studied. TABLE 1. RETENTION DATA FOR STANDARD SILYLATED MONOSACCHARDIES Using the retention data in table 1 (p. 299), the sugar components of each of the standard gum media samples were identified. The composition of the gums is shown in table 2 (p. 299). The greater the number of φ, the larger the proportion of a particular component in the sample. TABLE 2. SUGAR COMPONENTS IDENTIFIED IN STANDARD GUM MEDIA SAMPLES It has been reported the xylose and fucose are found together in significant levels only in gum tragacanth (around 13% and 4.5% respectively, expressed as relative percentage composition) and are minor components of gum ghatti (approximately 1.7% and 2.3% respectively). Significant levels of xylose, plus a small amount of fucose, in a sample would therefore indicate the presence of gum tragacanth (Ha and Thomas 1988). Xylose is found in low levels (around 4–6%) without fucose in cherry gum (Bleton et al. 1996). Arabinose is virtually undetectable in gum karaya, locust bean gum and guar gum, while mannose is the major component of both guar gum and locust bean gum. Hence, if a sample contained no arabinose or mannose, karaya gum would be the expected medium. Very high levels of mannose in the absence of arabinose would Gum arabic is indicated by the presence of relatively high levels of arabinose and rhamnose (approximately 18% and 11% respectively), while gums tragacanth and ghatti contain larger amounts of arabinose (22.4% and 31.6%) but much lower levels of rhamnose (between 6 and 6.5%) (Ha and Thomas 1988). Cherry gum contains a very large amount of arabinose (around 55%) with a negligible amount of rhamnose (between 2% and 3%) (Bleton et al. 1996). The uronic acids are not found in all of the common gum media studied. Galacturonic acid is found only in gums tragacanth and karaya, while glucuronic acid is a component of gums arabic, ghatti, karaya, and cherry (Ha and Thomas 1988, Bleton et al. 1996). Figure 1 (p. 300) shows the chromatograms obtained from the GC-MS analysis of standard gums arabic, tragacanth, cherry, and karaya, with peak retention times in minutes.
4.2 SAMPLES FROM WILLIAM BLAKE'S TEMPERA PAINTINGSSamples from tempera paintings by William Blake were analyzed, as part of an ongoing study into the artist's works. Blake's biographer Gilchrist (1863) described his “fresco” method, from ca. 1799, as involving the application of colors ground in “common carpenters' glue” to a very thick priming of glue and whiting: today this is described as “tempera.” Conclusions were drawn as to the nature of the binding media used by Blake in these particular works, using the results shown in table 3 (p. 305). TABLE 3. MONOSACCHARIDE COMPONENTS OF SAMPLES FROM PAINTING BY WILLIAM BLAKE The first painting studied was The Body of Christ Borne to the Tomb(fig. 2). The work, believed to be tempra on canvas, has previously undergone some retouching and consolidation with gelatine, the nature of which is currently unexamined, and exhibits a moderate amount of flaking and cracking. Samples of green paint and priming were removed and stored in glass vials prior to analysis.
The second painting studied, The Spiritual Form of Nelson Guiding Leviathan (fig. 3, p. 301), was also believed to be tempera on canvas. The work has undergone a moderate amount of retouching and consolidation with gelatine, again unexamined, but exhibits extensive flaking and cracking. Samples of white priming (possibly commerical oil priming with adsorbed paint medium from its appearance in cross section) and paint medium (including some varnish) had previously been removed and stored during conservation. These samples were submitted for gum analysis to avoid further sampling.
Bathsheba at the Bath(fig. 4) was again described as tempera on canvas. Retouching and consolidation with gelatine had been performed, though remains unexamined, while the work exhibits a moderate amount of flaking and cracking. Samples of priming (with some paint and varnish) and white and blue paint (with some varnish) were removed and stored prior to their analysis.
A fourth tempera work, The Ghost of a Flea (fig. 5, p. 303), has been varnished, but microscopical examination revealed no consolidation. The painting exhibits an extensive amount of flaking and cracking (below the present varnish layers). Samples of priming (with adsorbed paint medium), dark background paint at the lower left corner, and blue paint at the left edge were removed for analysis.
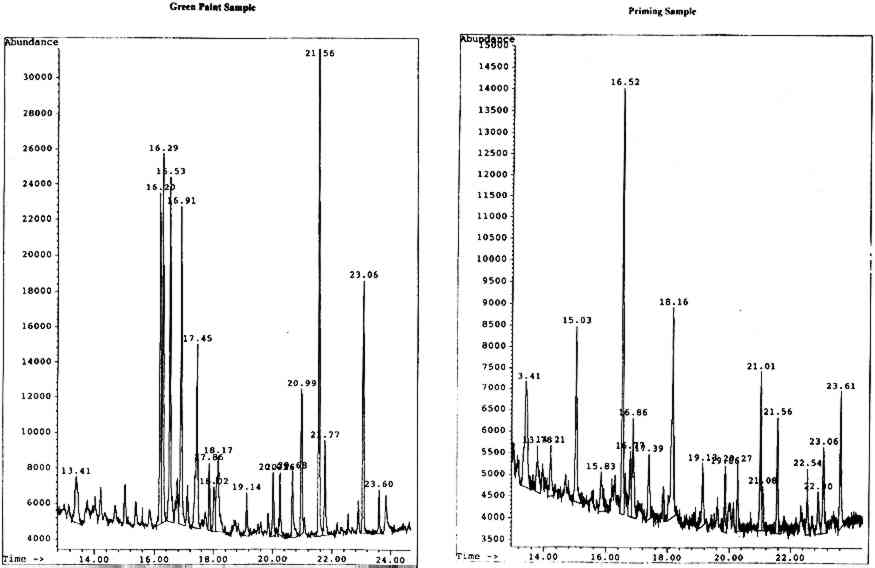
4.2.1 Body of Christ Borne to the TombOn analysis, the green paint was found to be a mixed gum medium, most likely containing gum arabic, gum tragacanth, and brown cane sugar, indicated by the presence of a significant amount of glucose in the sample. It is likely that the glucose results from the hydrolysis of the sucrose in cane sugar to its components, glucose and fructose. The priming appears to be predominantly karaya gum with added brown cane sugar, though the noticeable presence of xylose and fucose is perplexing in the apparent absence of gum tragacanth. The chromatograms for both the paint and priming samples are shown in figure 6 (p. 304).
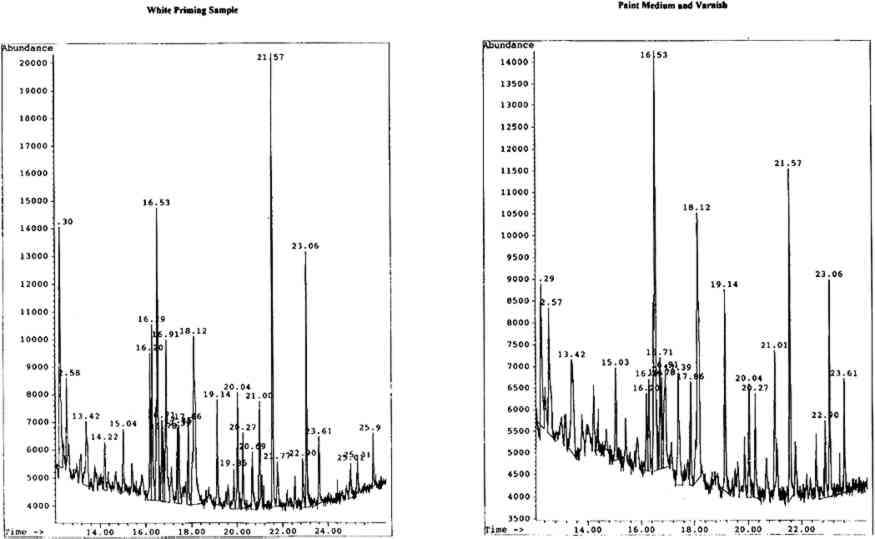
4.2.2 Spritual Form of Nelson Guiding LeviathanBoth the white priming and paint medium were mixed gum media comprised of gum karaya, gum tragacanth, and brown cane sugar. The very high levels of rhamnose (further enhanced by that from the gum tragacanth) suggest karaya gum, the lesser amounts of arabinose being from the gum tragacanth. An unidentified peak with a retention time of 12.57 minutes was found to be present only in the karaya gum standars. This peak was also detected in these particular samples. The chromatograms for both samples are shown in figure 7 (p. 304).
4.2.3 Bathsheba at the BathThe priming appears to be a mixture of gums arabic and tragacanth with added brown cane sugar, indicated by the presence of a significant amount of glucose in the sample. The white and blue paint sample was found to be predominantly brown cane sugar, glucose being by far the principal sugar component present. Small amounts of gums tragacanth and arabic were also detected. 4.2.4 The Ghost of a FleaOn analysis, the priming sample was found to contain only gum arbic. Both the dark background paint and blue paint samples appear to be mixtures of mainly gums tragacanth and arabic, with a smaller amount of karaya gum (indicated by the presence of a peak at approximately 12.57 minutes, seen only in standard karaya gum samples) and some added brown cane sugar. 4.2.5 SummaryVery few comparable analyses of gums have been published because all of Blake's surviving temperas are in poor condition and have, therefore, been consolidated and/or varnished extensively in the past. This practice has led to contamination of the original medium, and analytical methods published previously have generally not been sufficiently sensitive for their application to submilligram samples of gum/protein mixtures. One analysis, by TLC, of a privately owned Blake color print (date unspecified) suggested that karaya gum was a more likely component than gums arabic or tragacanth (Essick 1980). To the author's knowledge, this has been the only other attempt to perform gum analysis on naturally aged Blake media. The subsequent detection of karaya gum in such a limited number of other works of British art from the same period. We hope to report on the findings of these further studies upon their completion in the near future. 4.3 DERIVATIZATION PROBLEMSSilylation is a versatile technique used to increase volatility of suitable analytes, thereby enhancing GC performance. There are, however, practical aspects that should be considered prior to derivatization of a sample. All silylating reagents and derivatives are particularly susceptible to hydrolytic attack by any moisture present in the reaction mixture, resulting in incomplete silylation. Trimethylsilyl derivatives are especially sensitive, more so than silylated derivatives, which possess a much more sterically hindered silicon atom. Nevertheless, the trimethylsilylation of aqueous solutions of hydroxy compounds has been achieved, using a great excess of derivatizing agent (Valdez 1985). In most cases the silylating agent alone is an adequate solvent, but sometimes an additional solvent is required in the reaction and for sample dilution prior to analysis. The selection of a solvent is crucial since any active hydrogens, including those of the solvent, will be silylated. For this Hexamethyldisilazane, the silylating agent employed for this investigation, was one of the earliest reagents used for silylation. There is usually no need for additional solvents in reactions with HMDS, since it is liquid at room temperature and possesses adequate solvating properties (Evershed 1993). Despite the fact that it is not one of the strongest silyl donors available, it was a suitable choice of reagent for the silylation of natural gum samples, as carbohydrate compounds are relatively easy to fully silylate. One problem with silylation of carbohydrates is the existence of multiple reaction products, resulting in complicated chromatograms (Evershed 1993). The multiple products yielded by monosaccharides arise due to the formation of anomers (a specific term referring to carbohydrate stereoisomers differing only in configuration at the hemiacetal carbon atom) and interconversion between pyranose and furanose rings. Interconversion of the anomers occurs via the open chain form of the sugar, while mutarotation results from the opening and closing of the ring. Five tautomeric forms (i.e., structural isomers that are directly interconvertible) are usually obtained for each single sugar—two pyranose, two furanose, and the open chain form—and because they possess slightly different physical properties, they are generally separated by GC. This interconversion can be minimized by the use of rapid and mild derivatization conditions. If silylation is the chosen method of derivatization, it is desirable to protect the keto group of the monosaccharides prior to silylation in order to prevent the formation of enol-TMS ethers. These are unstable and complicate the analysis by giving rise to multiple products that cannot be prepared quantitatively (Halket 1993). There appears to be a lack of repeatability in the derivatization of the uronic acids present in some of the gum samples. It has been reported that the uronic acid linkage may be more resistant to acid hydrolysis than the glycoside linkage of the neutral monosaccharides (Jones and Albersheim 1972). The carboxylic acid function may result in the stabilization of the linkage, thus the yields of uronic acids after acid hydrolysis may be lower than expected (Fazio et al. 1982). The hydrolyzed uronic acids become lactonized; the degree of lactonization is not reproducible (Blake and Richards 1968; Fazio et al. 1982). Methanolysis, a technique that yields methyl glycosides, is equally efficient for the hydrolysis of neutral sugar glycoside linkages but causes less degradation of the released sugar residues, especially important in samples containing uronic acids (Chambers and Clamp 1971; Chaplin 1982; Dierckxsens et al. 1983). |