THE DEVELOPMENT AND INITIAL APPLICATION OF A GAS CHROMATOGRAPHIC METHOD FOR THE CHARACTERIZATION OF GUM MEDIASARAH L. VALLANCE, B.W. SINGER, S. M. HITCHEN, & J. H. TOWNSEND
ABSTRACT—This article details the initial investigations into the development and application of a simple, “one-pot” hydrolysis and derivatization procedure for the characterization of natural gum-based artists' binding media. Samples were hydrolyzed under acid conditions, then silylated with hexamethyldisilazane (HMDS). Separation and analysis of the volatile monosaccharide derivatives were achieved by gas chromatography-mass spectrometry (GC-MS) with selected ion monitoring (SIM). A selection of standard gum media was analyzed using this method, and a small number of suspected gum media samples taken from William Blake's temperas were characterized thus: results frequently indicated the presence of mixed gum media (commonly containing gums tragacanth, karaya, and arabic) with added cane sugar. TITRE—D�veloppement et premi�res applications d'une m�thode de caract�risation de liants � base de gomme par chromatographie en phase gazeuse. R�SUM�—Cet article pr�sente les �tudes pr�liminaires sur le d�veloppement et l'application d'une proc�dure simple d'hydrolyse et de d�rivatisation pour caract�riser des liants naturels � base de gomme utilis�s dans l'art. Des �chantillons furent hydrolys�s en conditions acides, puis silylat�s avec de l'hexamethyldisilazane. La s�paration et l'analyse des d�riv�s volatils de monosaccharides furent compl�t�es par chromatographie en phase gazeuse coupl�e � la spectrom�trie de masse, avec un contr�le d'ions s�lectionn�s. Plusieurs �chantillons standards de liants gommeux furent analys�s � l'aide de cette m�thode, ainsi que quelques �chantillons pr�lev�s sur des d�trempes de William Blake, qu'on croyait �tre des liants � base de gomme. Les r�sultats indiquent souvent qu'il s'agit d'un m�lange de gommes (d'habitude les gommes arabique, d'adragante et de karaya), avec en plus du sucre de canne. TITULO—El desarrollo de una aplicaci�n inicial de un m�todo de cromatografa de gases para la caracterizaci�n de aglutinantes de gomas. RESUMEN—Este art�culo detalla las investigaciones iniciales para el desarrollo y aplicaci�n de un procedimiento simple, “de un solo caldero”, y el procedimiento de derivatizaci�n para la caracterizaci�n de materials aglutinantes naturales en base a gomas para artistas. Las muestras fueron hidrolizadas bajo condiciones �cidas, luego sililadas con hexametil disilazano. La separaci�n y an�lisis de los derivados vol�tiles de monosac�ridos se obtuvieron por CG-SM con monitoreo de i�n selectivo (MIO). Una selecci�n de aglutinantes est�ndard de goma fue analizada usando este m�todo, y varias muestras de aglitunantes de goma tomadas de t�mperas por William Blake fueron as� caracterizadas. Los resultados frecuentemente indicaron la presencia de aglutinantes de goma mezclados (comunmente conteniendo goma tragacanto, karaya, y ar�biga) con az�car de ca�a a�adida. 1 INTRODUCTIONIt is important that conservators are provided with detailed information regarding the exact nature of the materials used by artists to assist in devising safe treatment plans that accommodate the artists' intention for the appearance of their works. Specific knowledge of the nature of the media used in particular works may shed light on why some paintings are in better condition than others of similar age. This article reports the initial investigations into the development of a simple procedure for the analysis of gum-based binding media. Gums are a group of non-crystalline, polysaccharide materials that can be found in vegetable matter and are often exuded when a plant is “wounded.” They are water-soluble or water-dispersible compounds with complex composition, usually consisting of a number of surgars (e.g., galactose and mannose) plus their uronic acid derivatives (e.g., galacturonic acid) (Gettens and Stout 1966). The plant gums arabic (Church 1901; Merrifield 1966), tragacanth (Gettens and Stout 1966), and cherry (Doerner 1934; Gettens and Stout 1966; Mills and White 1994) have been used for many centuries as the principal media for watercolors, minitures, and manuscript illumination and, on occasion, as sizing materials; indeed there is documentary evidence to suggest that gum was used as a medium prior to the use of drying oils (Laurie 1911; Mills and White 1994). Honey, cane sugar solution, and glycerine have long been used as additives in aqueous media (Gettens and Stout 1966), preventing the extreme drying that results in the brittleness of these particular media. Today, dextrin is more usually used for this purpose. Karaya gum, the dried exudate of the native Indian tree Sterculia urens, has been used as a cheaper substitute for gum tragacanth; alternative names include Indian tragacanth, Indian gum, and Sterticula gum (Furia 1981). Other plant gums, including carob or locust bean, tamarind, cholla, plus plant resins like myrrh and olibanum, have found uses in an artistic context either for painting or papermaking. It is thought that the Egyptians used locust bean gum for binding mummy wrappings, and tamarind seed gum was the principal paint medium for Indian miniatures and murals (Mills and White 1994). 2 ANALYSIS OF NATURAL POLYSACCHARIDE MATERIALS2.1 DERIVATIZATION TECHNIQUES FOR GC ANALYSIS OF SUGARSChemists have employed a wide variety of analytical techniques for the characterization of carbohydrate compounds, probably the most widespread over recent years being gas chromatography (GC). Since GC is dependent on the volatility of the analytes, it is necessary for the sugars to undergo hydrolysis, followed by some form of derivatization reaction prior to analysis. Methods involving the formation of fully and partially methylated methyl glycosides, acetates, acetals, trimethylsilyl ethers, and more volatile alditol acetate derivatives of monosaccharides were popular for a time (McInnes et al. 1958; Bishop and Cooper 1960; Bishop 1964; Lehrfeld 1981; Blakeney et al. 1983). Today, however, trimethylsilyl (TMS) derivatives of carbohydrates are among the most widely used, primarily due to their high volatility and ease of preparation (Sweeley et al. 1963; Sullivan and Schewe 1977; Honda et al. 1979; Li et al. 1983; Twilley 1984). Chromatograms of sugar-based materials can be complicated by the presence of between 1 and 5 peaks for each monosacharide, arising from the existence of the Methods for the separation of neutral sugars in gums have been reported (Al-Hazmi and Stauffer 1986), but the uronic acid components were analyzed using additional spectrophotometric and differential GC techniques (Selvendran et al. 1979; Lehrfeld 1981). Neutral sugars and uronic acids were analyzed simultaneously as trimethylsilyl methyl glycosides: methanolysis of the uronic acids was performed, then the neutral sugars were trimethylsilylated (Ha and Thomas 1988). A similar method was used in a study of Auracaria bidwilli gum (Aspinall and Fairweather 1965; Aspinall and McKenna 1968). An obvious problem associated with the use of any of these methods for the analysis of gums used in works of art is the sample size. Many of these techniques will not be sufficiently sensitive for the sometimes sub-nanogram samples available to the conservation scientist. Nevertheless, there has been some progress in the analysis of gum media from art objects using GC techniques, usually in conjunction with thin layer chromatography (TLC): TLC itself is suitable for qualitative analysis of larger gum media samples (∼1 mg). 2.2 CHROMATOGRAPHIC ANALYSIS OF GUM MEDIA FROM WORKS OF ARTGC and TLC were utilized to analyze the trimethylsilyl derivatives of sugars resulting from the hydrolysis of samples of the surface coating and paint from a wooden Egyptian sarcophagus, dating from the 21st dynasty. Findings disclosed the presence of gum tragacanth and honey (Masschelein-Kleiner and Tricot-Marckx 1965; Masschelein-Kleiner et al. 1968). Gum arabic was identified as the medium of a 16th-century manuscript by TLC (Flieder 1968), while the use of gum tragacanth in the paint of three ancient Egyptian epitaphal stelae was revealed by TLC (Szyszko 1972). Birstein (1975) employed GC for his study on the problems associated with the binding media found in Asian wall paintings, and GC was the method chosen for the study of paintings found in the tomb of Nefertari at Luxor (Mora et al. 1990; Palet and Porta, 1990). Twilley (1984) published a report on the analysis and artistic applications of plant gums. Analysis of samples was achieved via a number of techniques, including GC of trimethylsilyl sugar derivatives. Erhardt et al. (1988) employed GC analysis of TMS-oxime derivatives for their studies of gum media, and, most recently, Bleton et al. (1996) reported on a GC method for the analysis of ink samples from ancient manuscripts. Derrick and Stulik (1990) used pyrolysis–gas chromatography (Py-GC) to characterize natural gums used in works of art. Gums arabic, tragacanth, guar, ghatti, and karaya all gave distinguishable and reproducible pyrograms, enabling their identification. As part of a wider project investigating the media used by William Blake and other 19th-century British artists, we have developed a simple derivatization technique for the analysis of extremely small samples of gum media by GC-MS. The method of sample preparation, using HMDS with trifluoroacetic acid in pyridine, was originally employed for the silylation of syrups and concentrated aqueous solutions of sugars 3 EXPERIMENTAL3.1 SAMPLESInitially, specimens of standard monosaccharides and gum materials were studied in order to compile a library of standard chromatograms for comparison with samples from works of art. The monosaccharides used (arabinose, fucose, galactose, galacturonic acid, glucose, glucuronic acid, mannose, rhamnose, and xylose) were in chromatographically pure forms, and the gum materials used (arabic, tragacanth, cherry, guar, ghatti, locust bean, and karaya) were all in powdered form except arabic, which was obtained in both powdered and preprepared forms. Aqueous solutions (approximately 10%) of the gums were prepared (the gums being allowed to swell in water for 24 hours before filtering to remove insoluble material), painted onto glass microscope slides, and allowed to dry naturally for at least 1 week. One set of gum samples was used unaged, while the other set was artifically aged for comparative purposes. The samples were first subjected to light aging for 4 weeks at 18,000 lux using ultraviolet filtered, daylight color rendering fluorescent tubes (custom built), then thermally aged for 4 weeks at 60�C, 55% relative humidity in a climate-controlled oven. Replicates of these standad media, a minimum of 6 samples (both aged and unaged) from various sources, were analyzed in order to form a library of standard chromatograms to assist with identification of gum media removed from works of art. The samples investigated were from the collection of works by William Blake at the Tate Gallery, London. With the aid of a surgical scalpel, minute samples of paint were removed from the surface of works, taking care to include as little of the support as possible. Then the samples were immediately placed in chemically clean Reactivials, which were sealed with caps containing polytetrafluoroethene-coated septa. Standard gums (1–2 mg in Reacti-vials) and individual monosaccharide samples (<1 mg in Reacti-vials) were heated in a Reacti-Therm heating module at 105�C, for 30 minutes and 10 minutes respectively, with trifluoroacetic acid (0.1 ml, analytical grade, undiluted). The samples were allowed to cool to room temperature before pyridine (0.2 ml, analytical grade) was added to the vials. Hexamethyldisilazane (0.3 ml, derivatization grade) and trifluoroacetic acid (5 drops, analytical grade, undiluted) were added to the samples, which were vigorously shaken for 30 seconds and then allowed to stand for 1 hour prior to analysis. Samples removed from works of art (0.1 mm2 approximately) were placed directly into Reacti-vials then heated for 30 minutes at 105�C with trifluoroacetic acid (0.1 ml, analytical grade, undiluted) in a Reacti-Therm heating module. The samples were allowed to cool to room temperature before pyridine (0.1 ml, analytical grade) was added. Hexamethyldisilazane (0.2 ml, derivatization grade) and trifluoroacetic acid (3 drops, analytical grade, undiluted) were added to the samples, which were then shaken vigorously for 30 seconds and allowed to stand for 1 hour before being 3.2 ANALYSIS3.2.1 InstrumentationA 5890 Series II Gas Chromatograph fitted with a quadrupole mass selective detector was used. The detector temperature was 280�C and the injector temperature was 250�C. The column was a DB-5 (length 30 m; internal diameter 0.25 mm; film 0.25 microns; Fisher Scientific), and helium was used as the carrier gas (pressure 2.5 psi). Program control and data processing were achieved using an MS-Chemstation with the G1034B software package. 3.2.2 MethodologySeparation and analysis were achieved using GC-MS. A completely splitless injection (12-minute solvent delay) of sample (1 ml) was made onto the column. The 43-minute temperature program had an initial setting of 100�C, held for 2 minutes, and was then ramped, at a rate of 6�C min−1, to a final temperature of 285�C, which was held for 10 minutes. Detection was achieved via selected ion monitoring (SIM). Parameters were as detailed below, the selected ions having been chosen as suitable for monitoring purposes after careful study of a large number of chromatograms obtaine for standard monosaccharides:
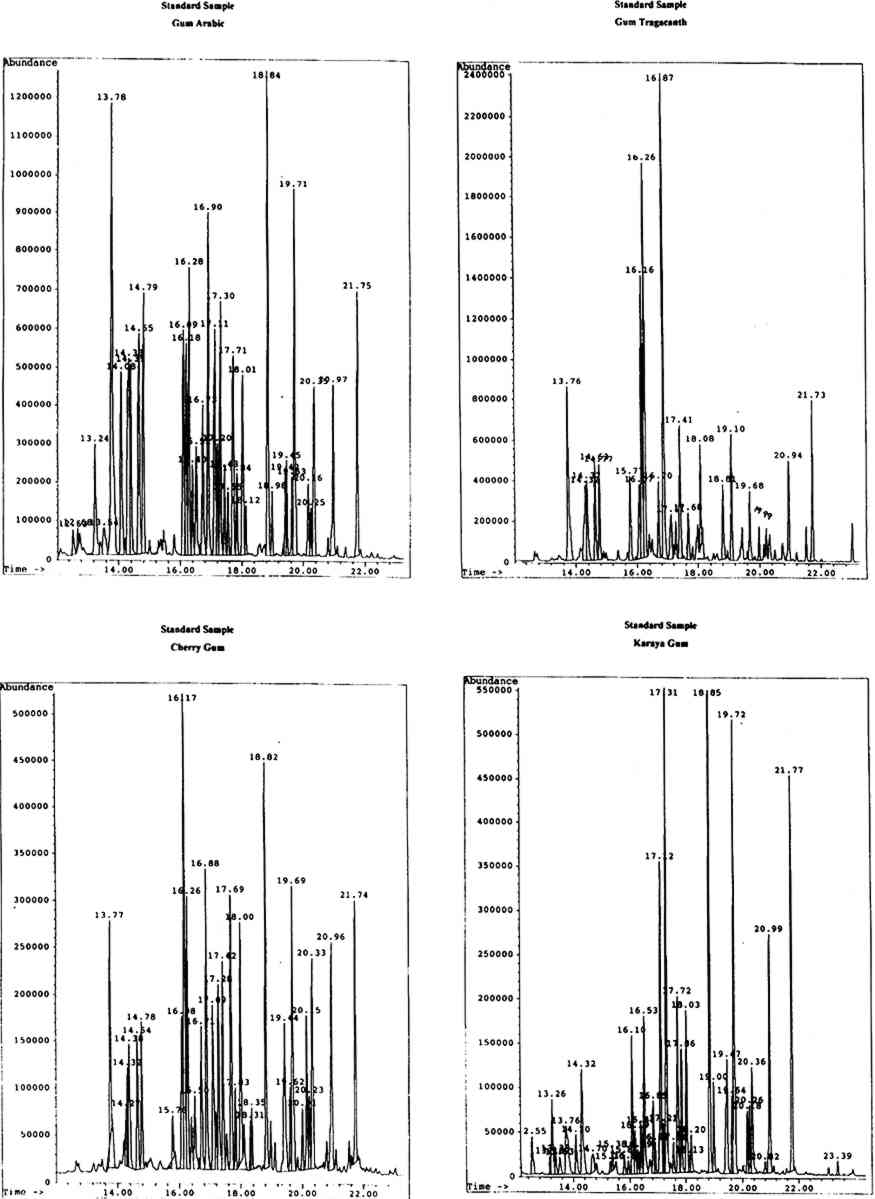
4 RESULTS AND DISCUSSIONThe identity of gums used as artists' media was ascertained by the analysis and identification of the monosaccharide components of the materials. Chromatographic data were first obtained for the standard monosaccharide samples, for comparison with that of the standard gum media. The chromatograms of the artificially aged and unaged standard gum media were very similar; hence the chromatograms obtained for the artificially aged gums were chosen to assist with identification of samples. 4.1 STANDARD GUM MEDIAResults for the individual standard monosaccharides will be considered first. A number of peaks were seen for each sugar, to a greater or lesser degree, corresponding to different structural forms of the silylated derivatives. table 1 shows the retention data for the individual monosaccharides studied. TABLE 1. RETENTION DATA FOR STANDARD SILYLATED MONOSACCHARDIES Using the retention data in table 1 (p. 299), the sugar components of each of the standard gum media samples were identified. The composition of the gums is shown in table 2 (p. 299). The greater the number of φ, the larger the proportion of a particular component in the sample. TABLE 2. SUGAR COMPONENTS IDENTIFIED IN STANDARD GUM MEDIA SAMPLES It has been reported the xylose and fucose are found together in significant levels only in gum tragacanth (around 13% and 4.5% respectively, expressed as relative percentage composition) and are minor components of gum ghatti (approximately 1.7% and 2.3% respectively). Significant levels of xylose, plus a small amount of fucose, in a sample would therefore indicate the presence of gum tragacanth (Ha and Thomas 1988). Xylose is found in low levels (around 4–6%) without fucose in cherry gum (Bleton et al. 1996). Arabinose is virtually undetectable in gum karaya, locust bean gum and guar gum, while mannose is the major component of both guar gum and locust bean gum. Hence, if a sample contained no arabinose or mannose, karaya gum would be the expected medium. Very high levels of mannose in the absence of arabinose would Gum arabic is indicated by the presence of relatively high levels of arabinose and rhamnose (approximately 18% and 11% respectively), while gums tragacanth and ghatti contain larger amounts of arabinose (22.4% and 31.6%) but much lower levels of rhamnose (between 6 and 6.5%) (Ha and Thomas 1988). Cherry gum contains a very large amount of arabinose (around 55%) with a negligible amount of rhamnose (between 2% and 3%) (Bleton et al. 1996). The uronic acids are not found in all of the common gum media studied. Galacturonic acid is found only in gums tragacanth and karaya, while glucuronic acid is a component of gums arabic, ghatti, karaya, and cherry (Ha and Thomas 1988, Bleton et al. 1996). Figure 1 (p. 300) shows the chromatograms obtained from the GC-MS analysis of standard gums arabic, tragacanth, cherry, and karaya, with peak retention times in minutes.
4.2 SAMPLES FROM WILLIAM BLAKE'S TEMPERA PAINTINGSSamples from tempera paintings by William Blake were analyzed, as part of an ongoing study into the artist's works. Blake's biographer Gilchrist (1863) described his “fresco” method, from ca. 1799, as involving the application of colors ground in “common carpenters' glue” to a very thick priming of glue and whiting: today this is described as “tempera.” Conclusions were drawn as to the nature of the binding media used by Blake in these particular works, using the results shown in table 3 (p. 305). TABLE 3. MONOSACCHARIDE COMPONENTS OF SAMPLES FROM PAINTING BY WILLIAM BLAKE The first painting studied was The Body of Christ Borne to the Tomb(fig. 2). The work, believed to be tempra on canvas, has previously undergone some retouching and consolidation with gelatine, the nature of which is currently unexamined, and exhibits a moderate amount of flaking and cracking. Samples of green paint and priming were removed and stored in glass vials prior to analysis.
The second painting studied, The Spiritual Form of Nelson Guiding Leviathan (fig. 3, p. 301), was also believed to be tempera on canvas. The work has undergone a moderate amount of retouching and consolidation with gelatine, again unexamined, but exhibits extensive flaking and cracking. Samples of white priming (possibly commerical oil priming with adsorbed paint medium from its appearance in cross section) and paint medium (including some varnish) had previously been removed and stored during conservation. These samples were submitted for gum analysis to avoid further sampling.
Bathsheba at the Bath(fig. 4) was again described as tempera on canvas. Retouching and consolidation with gelatine had been performed, though remains unexamined, while the work exhibits a moderate amount of flaking and cracking. Samples of priming (with some paint and varnish) and white and blue paint (with some varnish) were removed and stored prior to their analysis.
A fourth tempera work, The Ghost of a Flea (fig. 5, p. 303), has been varnished, but microscopical examination revealed no consolidation. The painting exhibits an extensive amount of flaking and cracking (below the present varnish layers). Samples of priming (with adsorbed paint medium), dark background paint at the lower left corner, and blue paint at the left edge were removed for analysis.
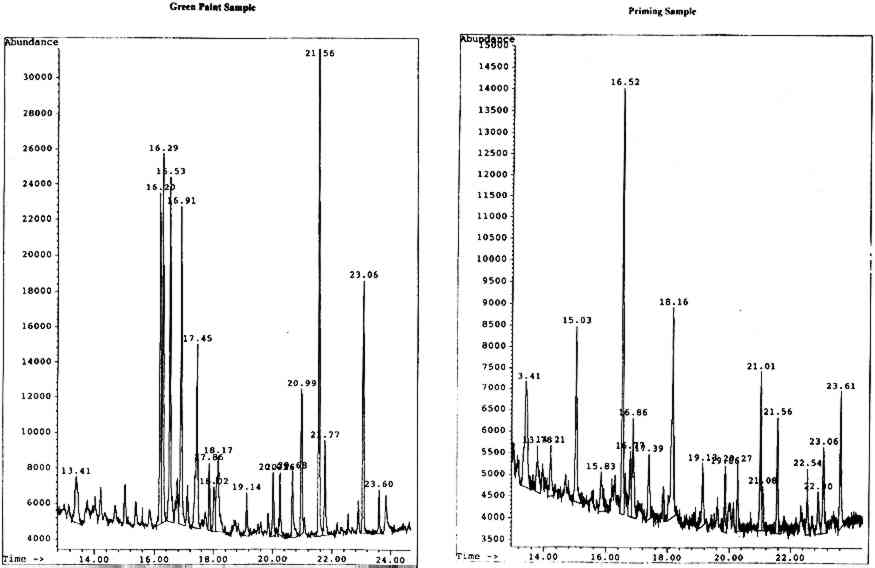
4.2.1 Body of Christ Borne to the TombOn analysis, the green paint was found to be a mixed gum medium, most likely containing gum arabic, gum tragacanth, and brown cane sugar, indicated by the presence of a significant amount of glucose in the sample. It is likely that the glucose results from the hydrolysis of the sucrose in cane sugar to its components, glucose and fructose. The priming appears to be predominantly karaya gum with added brown cane sugar, though the noticeable presence of xylose and fucose is perplexing in the apparent absence of gum tragacanth. The chromatograms for both the paint and priming samples are shown in figure 6 (p. 304).
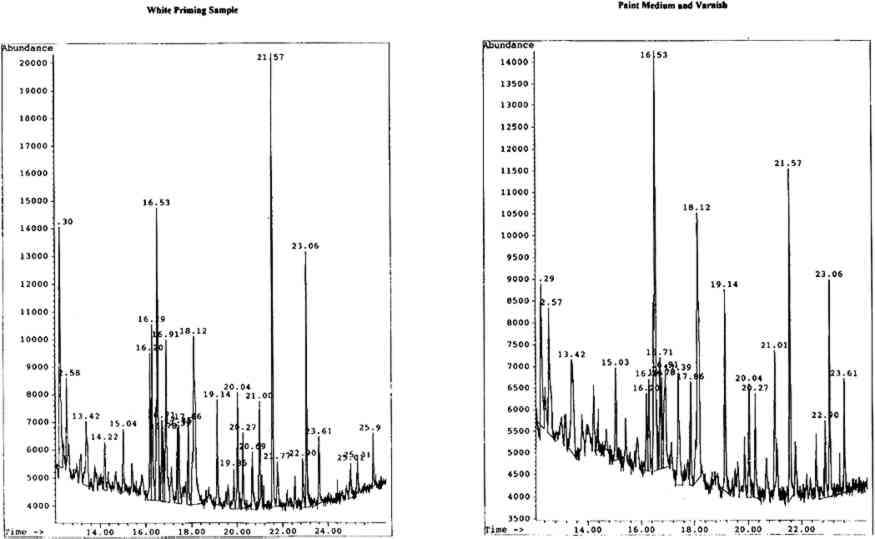
4.2.2 Spritual Form of Nelson Guiding LeviathanBoth the white priming and paint medium were mixed gum media comprised of gum karaya, gum tragacanth, and brown cane sugar. The very high levels of rhamnose (further enhanced by that from the gum tragacanth) suggest karaya gum, the lesser amounts of arabinose being from the gum tragacanth. An unidentified peak with a retention time of 12.57 minutes was found to be present only in the karaya gum standars. This peak was also detected in these particular samples. The chromatograms for both samples are shown in figure 7 (p. 304).
4.2.3 Bathsheba at the BathThe priming appears to be a mixture of gums arabic and tragacanth with added brown cane sugar, indicated by the presence of a significant amount of glucose in the sample. The white and blue paint sample was found to be predominantly brown cane sugar, glucose being by far the principal sugar component present. Small amounts of gums tragacanth and arabic were also detected. 4.2.4 The Ghost of a FleaOn analysis, the priming sample was found to contain only gum arbic. Both the dark background paint and blue paint samples appear to be mixtures of mainly gums tragacanth and arabic, with a smaller amount of karaya gum (indicated by the presence of a peak at approximately 12.57 minutes, seen only in standard karaya gum samples) and some added brown cane sugar. 4.2.5 SummaryVery few comparable analyses of gums have been published because all of Blake's surviving temperas are in poor condition and have, therefore, been consolidated and/or varnished extensively in the past. This practice has led to contamination of the original medium, and analytical methods published previously have generally not been sufficiently sensitive for their application to submilligram samples of gum/protein mixtures. One analysis, by TLC, of a privately owned Blake color print (date unspecified) suggested that karaya gum was a more likely component than gums arabic or tragacanth (Essick 1980). To the author's knowledge, this has been the only other attempt to perform gum analysis on naturally aged Blake media. The subsequent detection of karaya gum in such a limited number of other works of British art from the same period. We hope to report on the findings of these further studies upon their completion in the near future. 4.3 DERIVATIZATION PROBLEMSSilylation is a versatile technique used to increase volatility of suitable analytes, thereby enhancing GC performance. There are, however, practical aspects that should be considered prior to derivatization of a sample. All silylating reagents and derivatives are particularly susceptible to hydrolytic attack by any moisture present in the reaction mixture, resulting in incomplete silylation. Trimethylsilyl derivatives are especially sensitive, more so than silylated derivatives, which possess a much more sterically hindered silicon atom. Nevertheless, the trimethylsilylation of aqueous solutions of hydroxy compounds has been achieved, using a great excess of derivatizing agent (Valdez 1985). In most cases the silylating agent alone is an adequate solvent, but sometimes an additional solvent is required in the reaction and for sample dilution prior to analysis. The selection of a solvent is crucial since any active hydrogens, including those of the solvent, will be silylated. For this Hexamethyldisilazane, the silylating agent employed for this investigation, was one of the earliest reagents used for silylation. There is usually no need for additional solvents in reactions with HMDS, since it is liquid at room temperature and possesses adequate solvating properties (Evershed 1993). Despite the fact that it is not one of the strongest silyl donors available, it was a suitable choice of reagent for the silylation of natural gum samples, as carbohydrate compounds are relatively easy to fully silylate. One problem with silylation of carbohydrates is the existence of multiple reaction products, resulting in complicated chromatograms (Evershed 1993). The multiple products yielded by monosaccharides arise due to the formation of anomers (a specific term referring to carbohydrate stereoisomers differing only in configuration at the hemiacetal carbon atom) and interconversion between pyranose and furanose rings. Interconversion of the anomers occurs via the open chain form of the sugar, while mutarotation results from the opening and closing of the ring. Five tautomeric forms (i.e., structural isomers that are directly interconvertible) are usually obtained for each single sugar—two pyranose, two furanose, and the open chain form—and because they possess slightly different physical properties, they are generally separated by GC. This interconversion can be minimized by the use of rapid and mild derivatization conditions. If silylation is the chosen method of derivatization, it is desirable to protect the keto group of the monosaccharides prior to silylation in order to prevent the formation of enol-TMS ethers. These are unstable and complicate the analysis by giving rise to multiple products that cannot be prepared quantitatively (Halket 1993). There appears to be a lack of repeatability in the derivatization of the uronic acids present in some of the gum samples. It has been reported that the uronic acid linkage may be more resistant to acid hydrolysis than the glycoside linkage of the neutral monosaccharides (Jones and Albersheim 1972). The carboxylic acid function may result in the stabilization of the linkage, thus the yields of uronic acids after acid hydrolysis may be lower than expected (Fazio et al. 1982). The hydrolyzed uronic acids become lactonized; the degree of lactonization is not reproducible (Blake and Richards 1968; Fazio et al. 1982). Methanolysis, a technique that yields methyl glycosides, is equally efficient for the hydrolysis of neutral sugar glycoside linkages but causes less degradation of the released sugar residues, especially important in samples containing uronic acids (Chambers and Clamp 1971; Chaplin 1982; Dierckxsens et al. 1983). 5 CONCLUSIONSThese initial investigations have provided a simple derivatization method and GC technique for the characterization of natural gum-based artists' media, facilitating the identification of small and complex samples taken from temperas by William Blake. It appears that in the paintings studied, Blake used some unusual mixtures of plant gums and sugar. These findings have raised questions regarding the gums available to Hexamethyldisilazane proved an adequate silylating agent for the neutral sugars, though further work is needed to investigate the points raised in the discussion regarding the problem of the repeatability and reproducibility of the acid hydrolysis and silylation of uronic acids. One possibility would be to replace the acid hydrolysis stage of the sample preparation procedure with the alternative technique of methanolysis as used by Ha and Thomas (1988). ACKNOWLEDGEMENTSThe authors wish to thank Tate Publications for their permission to reproduce illustrations of Blake's works; Stephen Hackney, Dr. Tom Learner, and Roy Perry for reviewing the manuscript; and Ed Ludkin and Angela Pocklington for their technical assistance. REFERENCESAl-Hazmi, M. I., and K. R.Stauffer. 1986. Gas chromatographic determination of hydrolyzed sugars in commercial gums. Journal of Food Science51:1091–1092, 1097. Aspinall, G. O., and R. M.Fairweather. 1965. Araucaria bidwilli gum. Carbohydrate Research1:83–92. Aspinall, G. O., and J. P.McKenna. 1968. Araucaria bidwilli gum. Part 2. Further studies on the polysaccharide components. Carbohydrate Research7:244–54. Birstein, V. J.1975. On the technology of Central Asian wall paintings: The problem of binding media. Studies in Conservation20:8–19. Bishop, C. T.1964. Gas-liquid chromatography of carbohydrate derivatives. Advances in Carbohydrate Chemistry19:95–147. Bishop, C. T., and F. P.Cooper. 1960. Separation of carbohydrate derivatives by gas-liquid partition chromatography. Canadian Journal of Chemistry38:388–95. Blake, J. D., and G. N.Richards. 1968. Problems of lactonization in the analysis of uronic acids. Carbohydrate Research8:275–81. Blakeney, A. B., P. J.Harris, R. J.Henry, and B. A.Stone. 1983. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydrate Research113:291–99. Bleton, J., C.Coupry, and J.Sansoulet. 1996. Approche d'etude des encres anciennes. Studies in Conservation41:95–118. Brobst, K. M., and C. E.LottJr.1966. Determination of some components in corn syrup by gas-liquid chromatography of the trimethylsilyl derivatives. Cereal Chemistry43:35–43. Chambers, R. E., and J. R.Clamp. 1971. An assessment of methanolysis and other factors used in the analysis of carbohydrate containing materials. Biochemical Journal125:1009–1018. Chaplin, M. F.1982. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Analytical Biochemistry123:336–41.
Church, A. H.1901. The chemistry of paints and painting. 3d ed.London: Seeley and Co.79–80. Churms, S. C.1990. Recent developments in the chromatographic analysis of carbohydrates. Journal of Chromatography500:555–83. Decker, P., and H.Schweer. 1982. Gas-liquid chromatography on OV-225 of tetroses and aldopentoses as their O-methoxime and O-n-butoxime pertrifluoroacetyl derivatives and of C3-C6 alditol pertrifluoroacetates. Journal of Chromatography236:369–73. Derrick, M. R., and D. C.Stulik. 1990. Identification of natural gums in works of art using pyrolysis-gas chromatography. ICOM Committee for Conservation, preprints, 9th Triennial Meeting, Dresden, Paris: ICOM. 1:9–13. Dierckxsens, G. C., L. D.Meyer, and G. J.Tonino. 1983. Simultaneous determination of uronic acids, hexosamines, and galactose of galactosaminoglycans by gas-liquid chromatography. Analytical Biochemistry130:120–27. Dmitriev, B. A., L. V.Backinowsky, O. S.Chizhov, B. M.Zolotarev, and N. K.Kochetkov. 1971. Gas-liquid chromatography and mass spectrometry of aldononitrile acetates and partially methylated aldononitrile acetates. Carbohydrate Research19:432–35. Doerner, M.1934. The materials of the artist and their use in painting. New York: Harcourt, Brace. 223–24. Erhardt, D., W.Hopwood, M.Baker, and D.vonEndt. 1988. A systematic approach to the instrumental analysis of natural finishes and binding media. AIC preprints, American Institute for Conservation 6th Annual Meeting, New Orleans. Washington, D.C.: AIC. 7–84. Essick, R. N.1980. Appendix II. In William Blake: Printmaker. Princeton, N.J.: Princeton University Press. EvershedR. P.1993. Advances in Silylation. In Handbook of derivatives for chromatography, ed.K.Blau and J. M.Halket. Chichester, U.K.: Wiley. 53, 59, 70. Fazio, S. A., D. J.Ulinger, J. H.Parker and D. C.White. 1982. Estimations of uronic acids as quantitative measures of extracellular and cell wall polysaccharide polymers from environmental samples. Applied Environmental Microbiology43:1151–1159. Flieder, F.1968. Mise au point des techniques d'identification des pigments et des liants inclus dans la couche picturale des enluminures de manuscrits. Studies in Conservation13:49–86. Furia, T. E., ed.1981. Tree exudates and extracts: Gum karaya. In CRC handbook of food additives, 2d ed.Boca Raton, Fla.: CRC Press. 1:314–15. Gettens, R. J., and G. L.Stout. 1966. Gums. In Painting materials: A short encyclopaedia. New York: Dover. 13, 28–9. Gilchrist, A.1863. Life of William Blake. London and Cambridge: MacMillan. 69–70, 368–9. Ha, Y. W., and R. L.Thomas. 1988. Simultaneous determination of neutral sugars and uronic acids in hydrocolloids. Journal of Food Science53 (2):574–577. Halket, J. M.1993. Derivatives for GC-MS. In Handbook of derivatives for chromatography, ed.K.Blau and J. M.Halket. Chichester, U.K.: Wiley. 304.
Honda, S., K.Kakehi, and K.Okada. 1979. A convenient method for the gas chromatographic analysis of hexosamines in the presence of neutral monosaccharides and uronic acids. Journal of Chromatography Jones, J. M., and P.Albersheim. 1972. A gas chromatographic method for the determination of aldose and uronic acid constituents of plant cell wall polysaccharides. Plant Physiology49:926–36. Laurie, A. P.1911. The materials of the painter's craft. Philadelphia: Lippincott. 164. Lehrfeld, J.1981. Differential gas-liquid chromatography method for determination of uronic acids in carbohydrate mixtures. Analytical Biochemistry115:410–18. Li, B. W., P. J.Schuhmann, and J. M.Holden. 1983. Determination of sugars in yoghurt by gas-liquid chromatography. Journal of Agricultural and Food Chemistry31:985–89. Long, A. R., and G. W.ChismIII. 1987. A rapid direct extraction-derivatization method for determining sugars in fruit tissue. Journal of Food Science52:150–54. Masschelein-Kleiner, L., J.Heylan, and F.Tricot-Marckx. 1968. Contribution � l'analyse des liants, adh�sifs et vernis anciens. Studies in Conservation13:105–21. Masschelein-Kleiner, L., and F.Tricot-Marckx. 1965. La d�tection de polysaccharides dans les mat�riaux constitutifs des oevres d'art. Institut Royal du Patrimoine Artistique, Bulletin8:180–91. McInnes, A. G., D. H.Ball, F. P.Cooper, and C. T.Bishop. 1958. Separation of carbohydrate derivatives by gas-liquid partition chromatography. Journal of Chromatography1:556–57. Merrifield, M. P.1966. Original treatises on the arts of painting. New York: Dover. 1:284. Mills, J. S., and R.White. 1994. Cherry gum, carob/locust gum and tamarind mucilage. In The organic chemistry of museum objects. 2d ed.London: Butterworth Heinemann. 77, 78. Mora, P., L.Mora, and E.Porta. 1990. Conservation et restauration de la tombe de N�fertari dans la Vall�e des Reines. ICOM Committee for Conservation preprints, 9th Triennial Meeting, Dresden. Paris: ICOM. 518–23. Palet, A., and E.Porta. 1990. An�lisis qu�mico de los pigmentos y aglutinantes empleados en las pinturas murales de la tumba de Nefertari. Congresa de Conservaci�n de Bienes Culturales, Valencia, 20–23 Setiebre de 1990, 452–60. P. Roig Picazo. Selvendran, R. R., J. G.March, and S. G.Ring. 1979. Determination of aldoses and uronic acid content of vegetable fibre. Analytical Biochemistry96:282–92. Sullivan, J. E., and L. R.Schewe. 1977. Preparation and gas chromatography of highly volatile trifluoroacetylated carbohydrates using N-methyl bis[trifluoroacet-amide]. Journal of Chromatographic Science15:196–97. Sweeley, C. C., R.Bentley, M.Makita, and W. W.Wells. 1963. Gas-liquid chromatography of trimethylsilyl derivatives of sugars and related substances. Journal of the American Chemical Society85:2497–2507. Szyszko, W.1972. Technological investigations of the three Egyptian epitaphial stelae on wood supports now preserved in national museum, Krac�w, part 1. Ochrona Zabytkow25:170.
Twilley, J. W.1984. The analysis of exudate plant gums in their artistic applications: An Valdez, D.1985. Silylation of dilute hydroxy compounds in aqueous solutions. Journal of Chromatographic Science23:128. SOURCES OF MATERIALSStandard monosaccharides; pyridine [27,040-7]; 1,1,1,3,3,3-hexamethyldisilazane [H1,000-2]; trifluoroacetic acid [30,203-1]:Aldrich Chemical Company, Ltd., The Old Brickyard, New Rd., Gillingham, Dorset SP8 4JL, U.K. Standard gum media:A. P. Fitzpatrick, 1 Barnabas Studios, 10-22 Barnabas Rd., London, E9 5SB, U.K. Winsor and Newton, Whitefriars Ave., Harrow, Middlesex HA3 5RH, U.K. HP5890 Gas Chromatograph with Mass Selective Detector and HP 5895 GC Chemstation; DB-5 capillary column, 0.25 mm ID, 0.25 μm film thickness, 30 m lengthHewlett-Packard Ltd., Heathside Park Rd., Cheadle Heath, Stockport, Cheshire SK3 0RB U.K. Reacti-therm heating module; 1 ml Reactivials with screw caps; septaPierce and Warriner, 44 Upper Northgate St., Chester CH1 4EF, U.K. Custom-built climate-controlled oven for artificial agingFisher Scientific UK, Bishop Meadow Rd., Loughborough, Leicestershire LE11 0RG, U.K. AUTHOR INFORMATIONSARAH L. VALLANCE received her B.Sc. (Hons) in applied chemistry from the University of Northumbria at Newcastle in 1992. After a period in industry as a method development analytical chemist, she returned to the University of Northumbria at Newcastle in 1994 to begin Ph.D. studies in collaboration with the Tate Gallery, London. Her research focused on development and application of gas and high performance liquid chromatographic methods for identification of artists' proteinaceous and natural gum binding media, ultimate aim of project being study of late-18th- and early-19th-century works by artists such as William Blake, J.M.W. Turner, and D. G. Rossetti. She was awarded her Ph.D. in 1997 on “The Development and Application of Chromatographic Techniques in the Characterization of Artists' Media.” She has published a number of papers on work in conservation science. Currently, she is the assistant web editor at the Royal Society of Chemistry, London, U.K. Address: Royal Society of Chemistry, Burlington House, Piccadilly, London W1V OBN, U.K. BRIAN SINGER obtained his Ph.D. in the field of synthetic organic chemistry at the University of Leeds, U.K. A lectureship at STEPHEN M. HITCHEN graduated from the University of Manchester Institute of Science and Technology (UMIST) in 1976, with a B.Sc. (Hons) in chemistry. He obtained his Ph.D. in synthetic organic chemistry from UMIST in 1980 and is currently a senior lecturer in the Department of Chemical and Life Sciences at the University of Northumbria at Newcastle. His current research interests include aided method development in chromatography. Address as for Singer. JOYCE H. TOWNSEND received her B.Sc. (Hons) in physics from the University of Glasgow in 1979. She was a conservation scientist at Glasgow Museums, 1979–87, specializing in accelerated aging and materials testing for conservation. From 1987 to 1991, she was a conservation scientist, and from 1991 to the present, senior conservation scientist, at the Tate Gallery, London. She received her Ph.D. from the Courtauld Institute of Art, University of London, 1991, on “The materials and Techniques of JMW Turner, RA 1775–1851.” She concentrates on the identification and aging of artists' materials used in the later 18th and 19th centuries and also works with conservators who are evaluating or developing conservation processes. She has published studies on J.M.W. Turner, James Abbott McNeil Whistler, and Edgar Degas and is currently working on Sir Joshua Reynolds. She has been editor and technical editor of several conference proceedings, most recently Turner's Painting Techniques in Context (UKIC, 1995) and Resins: Ancient and Modern (SSCR 1995). Address: Conservation Department, Tate Gallery, Millbank, London SW1P 4RG, U.K.
 Section Index Section Index |