MOISTURE RELATIONSHIPS OF PHOTOGRAPHIC FILMP.Z. ADELSTEIN, J.-L. BIGOURDAN, & J.M. REILLY
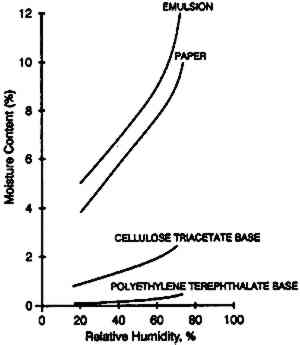
1 INTRODUCTIONThe moisture content of photographic film is one of the most critical characteristics that determines physical properties and practical behavior (Calhoun 1944). If the moisture content is high, photographic film may be tacky, be susceptible to mold growth, and will have reduced chemical stability (Adelstein et al. 1992b). If the moisture content is low, the stability will be increased, but the film may have susceptibility to static, be brittle, and show high curl; if it has a weak bond, it may exhibit emulsion-base adhesion problems. Dimensional changes as well as distortions that can develop in film rolls (Carver et al. 1943) are strongly influenced by the moisture content. Moisture content of films has always been evaluated and compared by the moisture equilibrium curve—that is, a plot of the weight percent of water
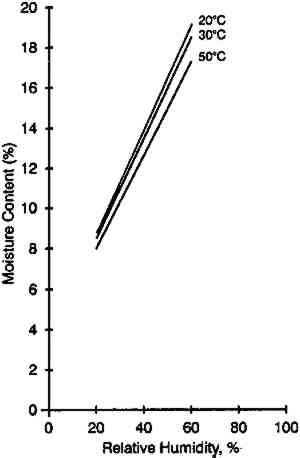
A main thrust of this article is a study of the effect of temperature on the moisture equilibrium curves of photographic film and its implications in both storage and laboratory situations. Limited data were reported by Adelstein et al. (1970) on a motion picture film on cellulose triacetate base. It was pointed out that although the moisture content of air at 49�C and 50% RH is 264 grains/lb. dry air and at 7�C and 50% RH is 22 grains/lb. dry air, the water content of this film was very similar despite the tenfold difference in water content of the air. There have been several more recent studies on the temperature effect since the 1970 publication. Iwano (1994) presented data on gelatin to a subcommittee of the American National Standards Institute (ANSI). A gravimetric procedure was used experimentally. This information, reproduced in figure 2, also shows a relatively low temperature dependence on the percent moisture content.
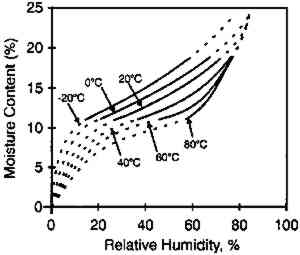
McCormick-Goodhart (1994) produced results of a similar study using a different laboratory procedure that was also presented at an ANSI meeting and subsequently published (1995, 1996). He first
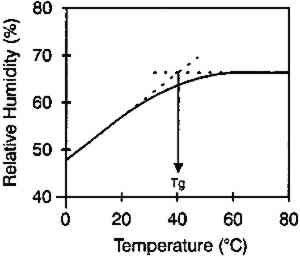
The moisture content isolines also revealed additional information about the hygroscopic behavior of gelatin. It can be observed from figure 3 that there was a general linear relationship between the equilibrium relative humidity and the temperature up to a temperature of 40�C. Above this temperature, there was very little change in the equilibrium relative humidity level. The explanation for this behavior is that 40�C is the glass transition temperature (Tg) of this gelatin. (The glass transition temperature is the temperature at which there is a change in the physical characteristics of amorphous materials. Gelatin is glassy in behavior below Tg and rubbery above Tg.) This procedure was used to measure the glass transition temperature, and the values listed by McCormick-Goodhart (1994) in table 1 compare favorably with those previously reported in the literature (Rose 1977). TABLE 1 MOISTURE PROPERTIES OF PHOTOGRAPHIC GELATIN This article presents additional data on the temperature effect on the moisture equilibrium curve. However, unlike the work of Iwano (1994) and McCormick-Goodhart (1994, 1995, 1996), who studied the gelatin binder, this study presents information on the complete photographic film on cellulose triacetate base. Iwano interpreted his data as it pertains to the humidity inside sealed bags when used in accelerated incubation tests. McCormick-Goodhart addressed this application as well as implications for the cold storage of photographic film. Both aspects will be discussed here. A consideration for cold storage of photographic A very practical aspect of moisture effects in storage is the moisture protection provided by various types of enclosure materials such as bags, boxes, and cans. Comparisons were made at both room temperature and at −16�C. |