A TRANSLUCENT WAX-RESIN FILL MATERIAL FOR THE COMPENSATION OF LOSSES IN OBJECTSSUSANNE G�NSICKE, & JOHN W. HIRX
ABSTRACT—A thermoplastic synthetic wax-resin mixture was developed by John W. Burke and Steve Colton for compensating losses in objects made from translucent materials. This material is an aesthetically pleasing and reversible alternative to other compensation materials such as epoxy and polyester resins or waxes. The authors describe how the various components of the mixture, including polyvinyl acetate (PVAC) AYAC, ethylene acrylic acid copolymers A-C 540 and 580, antioxidants Irganox 1076 or 1035, and a variety of possible fillers can be melted together, applied to areas of loss, and sculpted to shape. TITRE—Un mat�riel translucide compos� de r�sine et de cire pour combler les pertes sur les objets. R�SUM�—John W. Burke et Steve Colton ont mis au point un m�lange thermoplastique synth�tique pour combler les lacunes sur des objets translucides. Ce mat�riel, compos� de cire et de r�sine, est r�versible et esth�tique et constitue une alternative par rapport � d'autres mat�riaux de comblement tels que les cires ou les r�sines �poxydes et polyester. Les auteurs d�crivent comment les diff�rentes composantes du m�lange polyvinyl ac�tate (PVAC) AYAC, copolym�res et d'acide d'�thyl�ne acrylique A-C 540 et 580, antioxidants Irganox 1076 ou 1035 et une vari�t� de mat�riaux de charge), peuvent �tre fondus ensemble, appliqu�s � l'entroit de la lacune, et sculpt�s � la forme d�sir�e. T�TULO—Un material de relleno translucido de cera y resina para compensaci�n de faltantes en objetos. RESUMEN—Una mezcla termopl�stica sint�tica de cera y resina fue desarrollada por John W. Burke y Steve Colton para compensar los faltantes en objetos hechos de materiales transl�cidos. Desde el punto de vista est�tico este material es apropiado. Es una alternativa reversible a otros materiales de compensaci�n utilizados tales como resinas ep�xidas y de poliester o ceras. Los autores describen como los diferentes componentes de la mezcla, incluyendo acetato de polivinilo (PVAC) AYAC, copolimeros de �cido etilenacrilico A-C 540 y 580, los antioxidantes Irganox 1076 o 1035, y una variedad de posibles rellenos, pueden ser fundidos al tiempo. Esta mezcla puede ser aplicada a las �reas donde hay faltantes y luego esculpida para darle la forma necesaria. 1 INTRODUCTIONThe compensation of losses in objects made from translucent materials such as alabaster, marble, calcite, diorite, and anhydrite has always been challenging. Traditionally waxes, polyester, epoxy, and acrylic resins bulked with a variety of materials and coloring agents to imitate stone have been applied to areas of loss, but to date no satisfactory solution has been found. Waxes tend to collect dust and dirt. Both polyester resins and epoxies are toxic and noxious. If applied directly to the area of loss, they can migrate into and catalyze within the stone. With time, both materials will yellow and can lead to internal staining. These resins are difficult to remove; they only swell in solvents rather than dissolving, and swelling can cause cracking and delamination of the object. The application of lowviscosity synthetic resins sometimes requires the creation of complicated molds. If multicolored or banded fills are required, the work becomes even more complicated. About 10 years ago John Burke, Oakland
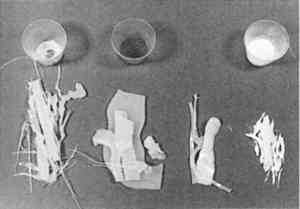
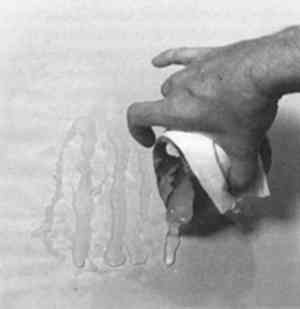
2 COMPONENTS OF THE FILL MATERIAL AND THEIR PROPERTIESPolyvinyl acetate resins are used variously by conservators as adhesives and varnishes (Horie 1987). PVAC AYAC is the primary binder and adhesive in the fill mixture. It is clear and photochemically relatively stable, and it has a molecular weight of 13,000 (Union Carbide Corp. 1982, 1989). Its primary industrial application is as a chewing gum elastomer (Reid 1994). It is soluble in a variety of organic solvents and has well-documented reversible characteristics that have been reviewed (Feller et al. 1978). The softening point of PVAC AYAC is 160�F (71�C), and it must be heated above 450�F (230�C) before it will begin to char. This resin, like the other PVAC resins, does not have a specific melting point; rather, it softens as the temperature rises until it eventually becomes a viscous flow. This characteristic is based on the fact that PVAC resins are blends rather than a specific molecule with a specific melting point (Reid 1994). Their glass transition temperature (Tg), which has been defined as “the temperature at which a material changes from a solid, glassy state to a softer rubbery state” (Schilling 1989, 110), is about 61�F (16�C). The ethylene acrylic acid copolymers are materials of low molecular weight (2000–2500) that are used as additives in adhesives and coatings and in hot-melt adhesives (Domine and Schaufelberger 1977). They are also emulsifiable in water-based systems (Huff 1995). The softening points are below 221�F (105�C) for A-C 540 and 203�F (95�C) for A-C 580 (Allied Signal Corp. 1993). These resins have some solubility in acetone; and there is no free acid present in the EAA copolymers. Their acrylic acid component gives these waxes increased toughness and better adhesion to polar substrates than PVAC resins. To the wax-resin they lend both translucency and toughness. At elevated temperatures polymers are susceptible to thermo-oxidative degradation; hot-melt adhesives are particularly prone to this There are two basic classes of stabilizers: primary or chain-terminating antioxidants and secondary hydroperoxide-decomposing antioxidants (Ciba Geigy Corp. 1990, 1993; Earhart et al. 1994). Primary antioxidants have also been described as radical scavengers because they react with peroxy and alkoxy radicals to form phenoxy radicals, which are relatively stable (de la Rie 1988a, 1988b). The Irganox 1076, a primary hindered phenolic antioxidant, was chosen for this formula because its melting point of 50–55�C is below the formulating temperature of the blend. Irganox 1035, another primary hindered phenolic antioxidant, has also been used in this formula. It has a sulfur bridge that provides peroxide decomposition capability in addition to standard antioxidant functionality. It is also a slightly larger molecule than Irganox 1076 and has a melting point of 145–54�F (63–68�C); but de la Rie (1988a) cautioned that the use of sulfur compounds, such as Irganox 1035, in metal ion-containing materials might lead to darkening. As Burke (1983) observed, the ratio of components can be altered according to the desired qualities of the fill material. He increased the PVAC AYAC content to harden the blend and to increase gloss, melting point, and viscosity. He noticed that increasing the EAA content increases the waxiness of the blend but also decreases the cold flow while simultaneously decreasing the melt viscosity. A-C 580 is softer and more adhesive than the A-C 540; varying the 540/580 proportion, therefore, affects those properties. Burke also experimented with adding an ultraviolet (UV) absorber, which appeared to prevent yellowing, but the UV absorber (Uvinul D-49) he used was strongly colored and imparted a slight tint of its own. 3 PRELIMINARY NOTES ON AGING PROPERTIES OF THE FILL MATERIALDegradation of polymers is complex and depends on numerous factors (McNeill 1992). To date we have observations only on color fastness and physical stability of the fill material;2 we also have some indication of whether or not the wax-resin shows cold flow relative to the individual components. The PVAC component in the wax-resin has been shown to be a relatively stable material and was classified by Feller (1978) as a class A material.3 When Burke conducted the blue-wool test on the EAA A-C 540, he found that it actually bleached slightly. A recent article by Down et al. (1996) describes PVAC AYAC as having a “fair resistance to yellowing,” but also confirmed its emission of small amounts of acetic acid. We do not know the glass transition temperature of the fill material, but the combination of polymers probably affect their respective Tgs. The PVAC's Tg goes up slightly, and the EAA's melting point goes down. It has been suggested that fillers such as pigments and marble powder may behave like tiny hurdles that the polymers have to move around, which slow them down (Schlumpf 1990). While one very large fill of the fills described below has changed its shape, all the others have remained apparently unchanged over the course of a decade. To further investigate the Tg of the material, a batch of pure PVAC AYAC and a batch of the fill material were made in the Objects Conservation Laboratory at LACMA and placed in dated containers on January 10, 1994, where they are being monitored. The PVAC has already begun to move, but the wax-resin has not. Another test being conducted at LACMA with a starting date of April 15, 1995, involves placing a 50 gm weight on a block of wax-resin to see if it displaces the resin. No movement has occurred to date (July 12, 1996). Certainly the ongoing tests described above are rudimentary, and many more tests will have to be done to examine the possible degradation of the material. 4 USE OF THE WAX-RESIN FOR TREATMENTS4.1 PREPARATION OF THE MATERIALThe following formula has been used for numerous treatments in several conservation laboratories: 9 parts PVAC AYAC, 3 parts A-C 540, 1 part A-C 580, and 0.5% by weight Irganox 1076 or 1035. Starting with the PVAC, these materials are melted together on a hot plate or wax pot, or in a well-controlled oven, at the lowest possible temperature. When the PVAC is melted, the antioxidant and thermal inhibitor Irganox is added. The EAA resins are slowly stirred into the hot melt. The components are not completely miscible; rather, they are slightly incompatible. Discoloration and separation can occur if the temperature is too high or if the mixture is not stirred enough. Separation is easily recognizable, for the PVAC floats in translucent bubbles on the surface of the mixture; stirring will reverse this situation. If no pigments or fillers are added, the mix becomes a translucent, light grayish white, somewhat glassy material. Dry pigments can be added as needed (fig. 2). If the treatment requires the use of small strips of cold wax- resin, the melted mass can be poured into strips on Mylar or glass and allowed to cool (fig. 3).
4.2 EXAMPLES OF TREATMENT APPLICATION AND EVALUATION OF EXISTING FILLSThe break edges to which the fill is to be applied must be coated with a primer, which consists of a brushable solution of PVAC AYAA, 10–30% in organic solvents, wt./vol. This PVAC primer will soften as the warm wax-resin is applied to the object and will form a bond with the fill; without the primer the wax-resin fill will not adhere well to the object. In some The wax-resin can be manipulated in a number of different ways. Many types of losses can be filled using sticks of wax-resin softened with a hot-air gun (fig. 4) or a heated spatula, pressing the material into the primed area to be filled. Slight warming of the object, if at all possible, prevents cold joint. For example, an Egyptian Old Kingdom spouted jar (fig. 5) at the Museum of Fine Arts, Boston, carved from Egyptian alabaster (i.e., calcite), was broken in two pieces and had a loss at one side that was to be filled. After cleaning the fragments and mending the jar, sticks and sheets of the fill material, colored to match the base color of the jar, were melted against the primed break edges. As the material cooled slowly, more layers were added and the fill was smoothed with a heated spatula. The manipulation of the warm material, which has a toffeelike, slightly stringy consistency, is somewhat messy. Therefore it was easiest to overfill and continue final shaping mechanically. The wax-resin was sanded, buffed, and polished with a cotton cloth and Novus Plastic Polish 2 to match the surface luster of the original stone (fig. 6). A high gloss can be obtained by polishing a fill that has been made scratch free by sanding with increasingly fine sandpapers. As full compensation of the loss was required, acrylic emulsion paint was used to continue a dark stain and some superficial white spots from the original surface (fig. 7).
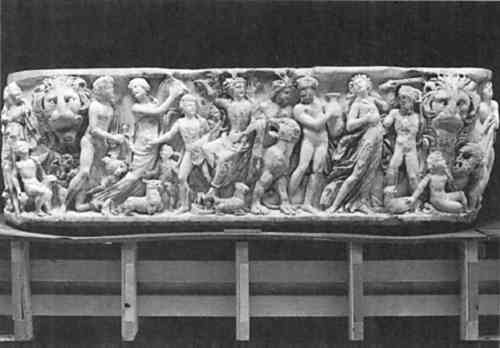
A second example for working the warm fill material into an area of loss is a Roman marble sarcophagus (fig. 8) at LACMA, which had numerous gaps (fig. 9) along old break edges that needed to be filled in 1982. The wax-resin was applied to the primed stone edges in numerous areas with a heat spatula. The fills have been monitored since and have shown no signs of
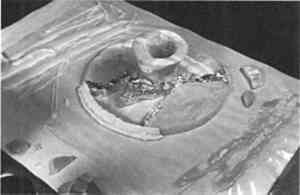
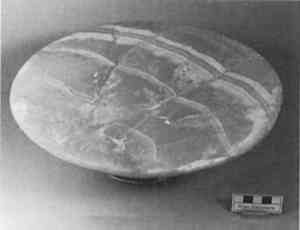
Sometimes a loss is so large that it requires separate construction and attachment with an adhesive different from the primer. Larger losses can be cast, as was done on an Egyptian Old Kingdom offering table (fig. 11), in preparation for a special exhibition on funerary art, Mummies and Magic, at the Museum of Fine Arts, Boston, in 1988. This object was carved from a single piece of calcite, which was characterized by bands of different colors, ranging from white to amber, and from opaque to translucent. The offering table was to be displayed elevated on an ancient ceramic stand, where light shining through the half-inch thick veined top was to display the quality of the stone.
The top of the offering table was broken into four pieces; one-third of it was lost and needed to be replaced. Plaster and other opaque materials would not fulfill the requirements for this display, and an alternative to polyester and epoxy was sought. A large amount of the wax-resin was prepared and colored to match the ochre base color of the stone. The fill was initially cast as a thick monochrome piece within the break area but was separated from the stone by aluminum foil (fig. 12). It was further molded with a hot spatula. To recreate the opaque white veining of the stone, grooves were carved into
These bands were slightly overfilled, and the cooled surface was then shaped with sharp tools and sandpaper. Given the large size and weight of the fill section, a bond formed by PVAC alone did not seem practicable. The fill, therefore, was adhered to the reconstructed stone table fragments with a two component epoxy resin (Epo-Tek 301–2) after the break edges had been coated with R�hm and Haas Acryloid B-72 (fig. 13). The object has been on display in the Museum of Fine Arts since 1988. While the color has not changed visibly, the fill has sagged noticeably (fig. 14). This example certainly reveals the limitations of this material. If the treatment were to be repeated, perhaps it might benefit from a laminated fill incorporating a translucent core of another, more rigid, material or dowels, to which the wax-resin would be applied.
Another limitation of the material is that the wax-resin cannot mask underlying dark materials. This problem was encountered during the treatment of a group of 19th-century white marble sculptures recently acquired by the Chrysler Museum, Norfolk, Virginia. Many of the sculptures had been previously restored. Large losses had been compensated with plaster and/or polyester resins cast in place. The polyester used to fill the losses had aged to a dark greenish yellow color. Because it was cast onto the object, the resin migrated into and catalyzed within the stone, resulting in an irreversible interior dark band. Since the polyester fills could not be removed, they were mechanically pared down and recoated with the wax-resin to simulate more closely the appearance of the stone. The sculpture Ganymede and the Eagle by Bertel Thorwaldsen (fig. 15) had broad plaster compensations about the base of the eagle's feet that had crystallized in the stone, causing the break edges to become sugary and friable (fig. 16). The plaster fills were removed and replaced with removable subsurface fills of Pliacre Epoxy Putty, which is gray in color, to minimize the amount of the wax-resin that had to be applied. The Pliacre fills were surfaced with the wax-resin (figs. 17, 18).
In both instances where a dark underlying core existed, such as the old polyester fills or the detachable Pliacre Epoxy Putty subsurface fills, the darker core material will remain visible as a shadow inside, even if covered with a thick layer of wax-resin. This problem can be somewhat alleviated by using a white epoxy putty, such as Milliput Superfine Epoxy Putty, under the wax. Using the methods described above, any number of natural stones can be closely imitated. Differently colored and opaque patterns can be alternated to achieve effects of depth or to imitate granular or speckled stones. 5 CONCLUSIONSThis wax-resin mixture has been useful for the compensation of losses in translucent materials, especially stones such as alabaster, marble, calcite, diorite, and anhydrite. The relative ease and speed with which it can be applied makes it a particularly attractive choice. Because the use of the material is based on modeling and the addition of further layers, any possible shrinkage can be compensated for with the addition of more wax-resin. The fills remain easily removable at all times and do not penetrate the original material. The wax-resin is used best on losses that allow for a large contact with the original, primed surface and on losses that are thicker than approximately 1/16 in. Shallow losses and small gaps, for example between break edges, proved to be somewhat difficult, as the fill is easily pulled out during surface manipulation. Similarly, the wax-resin seems unsuited for large, unsupported fills. Experimentations with mixture ratio and with other resins may lead to improved results; and their aging properties have to be further investigated. ACKNOWLEDGEMENTSThanks to John Burke and Steve Colton for discussing their treatments and research and for supporting this publication. The late Jane Carpenter-Poliquin should be remembered especially for introducing the wax-resin to the Straus Center for Conservation and Technical Studies, Harvard University Art Museums. Carol Warner, National Park Service, Cultural Resources Center, Lowell, Massachusetts, was first to experiment with the material and to use it at the Museum of Fine Arts, Boston. Arthur Beale and Timothy Kendall, both of the MFA, and Pieter Meyers, Los Angeles County Museum of Art, helped to edit the paper. NOTES. The term “wax” in “wax-resin” is used throughout the article in reference to ethylene acrylic acid copolymer (EAA). “Wax” is a rather loose term applied to a variety of substances consisting of hydro-carbons and sharing a number of characteristics such as waxy feel. Furthermore, most of them are relatively solid at room temperature, liquefy with increasing temperatures, and will solidify when cooled again. NOTES. Burke has observed that some of the earliest samples he used have yellowed, a process he attributes either to lack of an antioxidant or to overheating at the time. He also subjected samples of the wax-resin to accelerated aging and then separated PVAC from EAA by melting. He noted that it appeared the PVAC component in fact had yellowed. Based on this observation, Union Carbide recommended adding a cycloaliphatic epoxy resin (ERL–4221) as a stabilizer to the PVAC. Samples of wax-resin prepared with this additive do not seem to have yellowed over 10 years. Burke has continued to use the epoxy resin as an antioxidant substitute for Irganox when he wanted to manipulate the hardness of the wax-resin. The cycloaliphatic epoxy resin is a liquid; Irganox is a solid. The inclusion of the epoxy resin results in a flexible wax-resin material. The addition of Irganox results in a material that is hard. The addition of either antioxidant is the same: 0.5% by weight. Burke is exploring other conservation applications of the EAA copolymers. NOTES. Feller published a grading system for conservation materials and their expected photo-stability based on the blue-wool test using the International Standard Organization R105 blue wool standard 6 for photochemical stability, in which the degree of fading of a sample of blue wool has been quantified. Under most conditions, conservators prefer to use a class A material, which, based on annual museum light levels, should last for 100 years or more or suffer no more than 20% loss of its essential properties. REFERENCESAllied Signal Corp.1993. Product specifications. Specifications 605800227 and 505400227. Burke, J.1983. Personal communication to Billie Milam and Steve Colton. 7969 Sunkist Dr., Oakland, Calif. 94605–3050. Ciba-Geigy Corp.1990. Antioxidants for polyolefins. A350B5M60. Ciba-Geigy Corp.1993Additives for coatings. A-288C7.5M23. de laRie, E. R.1988a. Polymer stabilizers: A survey with reference to possible applications in the conservation field. Studies in Conservation33: 9–22. de laRie, E. R.1988bStable varnishes for old master paintings. Diss. Netherlands: Krips Repro Meppel. Domine, J. D., and R. H.Schaufelberger. 1977. Ethylene copolymer based hot melt adhesives. In Handbook of Adhesives, ed.I.Skeist. New York: Van Nostrand Reinhold Company. 495–506.
Down, J. L., M. A.MacDonald, J. T.�treault, and R. S.Williams. 1996. Adhesive testing at the Canadian Conservation Institute: An evaluation of selected Earhart, N., A.Patel, and G.Knobloch. 1994. Thermal stabilization of adhesives. In Handbook of adhesive technology, ed. A.Pizzi and K. L.Mittal. New York: Marcel Dekker. 241–55. Feller, R.1978. Standards in the evaluation of thermoplastic resins. In ICOM Committee for Conservation preprints, 5th triennial meeting, Zagreb. Paris: ICOM. 16(4): 1. Feller, R., E. H.Jones, and N.Stolow. 1978. On picture varnishes and their solventsOberlin, Ohio: Intermuseum Conservation Association. Horie, C. V.1987. Materials for conservation. London: Butterworths. 92–96. Huff, M.1995. Personal communication. Allied Signal Corp., P.O. Box 2332, Morristown, N.J. 07962–2332. McNeill, I. C.1992Fundamental aspects of polymer degradation. In Polymers in conservation, ed. N. S.Allen, M.Edge, and C. V.Horie. Cambridge: Royal Society of Chemistry. 14–31. Reid, G.1994Personal communication. Technical consultant, Union Carbide Corp., Old Ridgebury Rd., Danbury, Conn. 06817. Schilling, M.1989The glass transition of materials used in conservation. Studies in Conservation34: 110–16. Schlumpf, H. P.1990. Fillers and reinforcement. In Plastic additives handbook: Stabilizers, processing aids, plasticizers, fillers, reinforcements, colorants for thermoplastics, ed. R.G�chter and H.M�ller. New York: Oxford University Press. 525–91. Union Carbide Corp.1982. Specialty polymers. F-49563. Union Carbide Corp.1989. Polyvinyl acetate resins for coatings and adhesives. SC–1127. SOURCES OF MATERIALSPolyvinyl acetate resins PVAC AYAA and AYACUnion Carbide Corp., Specialty Chemicals and Plastics, Old Ridgebury Road, Danbury, Conn. 06817 Ethylene acrylic acid copolymers A-C 540 and 580Allied Signal Corp. A-C Performance Additives, P.O. Box 2332, Morristown, N. J. 07962–2332 Irganox, antioxidant and thermal stabilizerCiba-Geigy Corp. Seven Skyline Dr. 3, Hawthorne, N.Y. 10532–2188 Mylar, polyester filmConservation Materials, Ltd. P.O. Box 2884, Sparks, Nev. 89431 Novus Plastic Polish 2Novus, Inc., 10425 Hampshire Ave. So., Minneapolis, Minn. 55438 Epo-Tek 301–2Epoxy Technology, Inc., 14 Fortune Dr., Billerica, Mass. 01821 Pliacre Epoxy PuttyPhiladelphia Resins Corp., 20 Commerce Dr., Montgomeryville, Pa. 18936 Milliput Superfine Epoxy PuttyMilliput Corp., Unit 5, The Marion, Dolgellau, Mid Wales LL40 1UU, United Kingdom R�hm and Haas, Independence Mall West, Philadelphia, Pa. 19105 AUTHOR INFORMATIONSUSANNE G�NSICKE has been an assistant conservator in the Department of Objects Conservation and Scientific Research at Museum of Fine Arts, Boston, since 1990. In 1987 she received a certificate in archaeological conservation from the R�misch-Germanisches Zentralmuseum, Mainz, Germany. She then served an advanced-level internship at the Museum of Fine Arts, followed by an Andrew W. Mellon Fellowship in Objects Conservation at the Metropolitan Museum of Art. She has worked as site conservator at the New York University Apis Expedition at Memphis, Egypt, and at the BMFA Expedition at Gebel Barkal, Karima, Sudan. She is particularly interested in the technical study of ancient Egyptian and Nubian material culture. Address: Objects Conservation and Scientific Research, Museum of Fine Arts, 465 Huntington Ave., Boston, Mass. 02115. JOHN W. HIRX is currently a research assistant in the Conservation Center of the Los Angeles County Museum of Art, where he pursues research on the preservation of archaeological and decorative silver. He was formerly a contract conservator at the Brooklyn Museum, treating the Egyptian collection prior to a reinstallation of the newly renovated galleries. Address: Conservation Center, Los Angeles County Museum of Art, 5905 Wilshire Blvd., Los Angeles, Calif. 90036.
 Section Index Section Index |