AQUEOUS LIGHT BLEACHING OF PAPER: COMPARISON OF CALCIUM HYDROXIDE AND MAGNESIUM BICARBONATE BATHING SOLUTIONSTERRY TROSPER SCHAEFFER, VICTORIA BLYTH-HILL, & JAMES R. DRUZIK
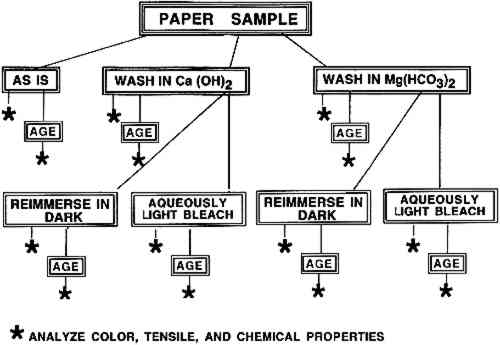
ABSTRACT—The immediate and long-term effects of aqueously light bleaching both unsized and gelatin-sized cotton cellulose papers in either calcium hydroxide, Ca(OH)2, or magnesium bicarbonate, Mg(HCO3)2, solutions were investigated. After samples were treated by washing, bleaching, or control bathing in the dark, one-half of each was artificially aged, and the condition of all samples was assessed. Appearance was analyzed by reflectance spectrometry, tensile behavior was observed using stress-strain measurements, and pH and gelatin content in the papers were measured with modified Tappi standard measurement procedures. the results showed that exposure to light per se during the bleaching process did not adversely affect the properties of the papers. It appeared that aqueous light bleaching of the unsized paper was equally effective in either solution. The visible changes in appearance, as well as alterations in other properties of the gelatin-sized paper, were influenced by the partial removal of sizing upon immersion. Finally, a clear preference for calcium hydroxide or magnesium bicarbonate as the bathing solution for aqueous light bleaching was not suggested by the results of the limited number of experiments possible in this study. Thus the choice of immersion solution should be made on an individual basis. TITRE—Blanchiment par lumi�re des papiers: Comparaison entre les bains de calcium hydroxyde et de magn�sium bicarbonate. R�SUM�—Il s'agit d'une �tude portant sur les effets imm�diats et � long terme du blanchiment par lumi�re sur des papiers en coton cellulose, � la fois non-encoll�s et encoll�s de g�latine, dans une solution de magn�sium bicarbonate Mg(HCO3)2 ou de calcium hydroxyde Ca(OH)2. Apr�s les traitements de lavage et blanchiment ou de lavage de control dans l'obscurit�, la moiti� de chaque �chantillon fut viellie artificiellement, et l'etat de tous les �chantillons fut �valu�. L'aspect fut analys� par spectrom�trie r�fl�chissante, la r�sistance � la tension fut observ�e a travers des mesures de tension-d�formation, le pH et la teneur en g�latine des papiers furent mesur�s au moyen de proc�dures TAPPI de mesures standards modifi�es. Les r�sultats ont d�montr� que l'exposition � la lumi�re pendant le blanchiment n'avait aucun effet n�gatif sur les propri�t�s des papiers. Il semblerait que le blanchiment par lumi�re des papiers non-encoll�s soit �galement efficace dans l'une ou l'autre des solutions. Les changements visibles de l'aspect ainsi que les modifications des autres propri�t�s du papier encoll� de g�latine sont influenc�s par la perte partielle de la colle d�s l'immersion du papier dans la solution. Enfin, les r�sultats des exp�riences ne sugg�rent pas une pr�f�rence nette pour le calcium hydroxyde ou pour le magn�sium bicarbonate comme solution de blanchiment par lumi�re. Donc, le choix de la solution d'immersion devrait �tre fait cas par cas. T�TULO—Blanqueo de papel por medio de 1 INTRODUCTIONWhen in 1980 Keyes first presented her conservation procedure of aqueous light bleaching to lessen discoloration of paper, she immersed the objects to be bleached in ca. 0.2% Mg(HCO3)2 solution (1987). Since that time, various concentrations of either Ca(OH)2 or Mg(HCO3)2 solutions have been used as immersion solutions (Baker 1982; Branchick et al. 1982; Eldridge 1982; van der Reyden et al. 1988; Lienardy and van Damme 1989; Schaeffer et al. 1992). Some conservators prefer one solution over the other. However, no direct comparison of aqueous light bleaching in Ca(OH)2 or Mg(HCO3)2 has been reported for controlled experiments in which long-term as well as immediate effects of the treatments were quantitated. We have now undertaken such a comparison, emphasizing the use of experimental conditions similar to those used by conservators in actual treatments. A gelatin-sized artists' paper and an unsized cellulose paper used as a control were each subjected to several treatments in this study. Half of every sample was aged artificially in a humid oven, so that direct comparisons could also be made of long-term effects of each treatment on the sample papers. Ca(OH)2 and Mg(HCO3)2 solutions are also employed by paper conservators as deacidification solutions (Lienardy and van Damme 1990). It has not been the intention of this investigation to compare the effectiveness of these two solutions for deacidification; a substantial literature already addresses this question (Hey 1979; Calvini et al. 1988; Bredereck et al. 1990; Lienardy and van Damme 1990). We have instead compared the efficacy of these two solutions for use as immersion baths for the aqueous light-bleaching process. The results of our experiments are presented below. No clear-cut preference for one immersion solution over the other emerged from the data, in part because the sized and unsized papers responded differently to the treatments. These results have also served to emphasize that some gelatin sizing is lost from papers when they are bathed and to illustrate the influence of this sizing material on some physical properties of the papers. 2 MATERIALS AND METHODS2.1 MATERIALSTwo papers with 100% cotton cellulose fiber were used for these experiments. The first was a heavily gelatin-sized artists' paper made by Whatman in 1956. It also contained alum. This paper, naturally aged for ca. 35 years, had been stored flat in the dark for the last several years. It was described in detail in an earlier publication (Schaeffer et al. 1992). Unsized Whatman 1 filter paper served as a control for the response of the α-cellulose fiber furnish alone to the treatments. Neither paper had disfiguring stains or noticeably uneven discolorations. The preparation of the Ca(OH)2 and Mg(HCO3)2 immersion solutions used in the treatment procedures is described in appendix A. Solutions at these or similar concentrations 2.2 EXPERIMENTAL PROTOCOLThe experimental plan was designed to permit a direct comparison of the effect of each treatment on the two different papers. The handling and conservation treatments of the paper samples were similar to those that conservators apply to works of art on paper. The procedures that mimicked conservation treatments were washing in dilute Ca(OH)2, aqueous light bleaching of the washed paper in Ca(OH)2, washing in Mg(HCO3)2, and aqueous light bleaching of the washed paper in Mg(HCO3)2. The control procedure of reimmersing a washed paper sample in fresh bathing solution in the dark was also performed to distinguish any changes due to exposure to light from those due solely to the additional immersion step. The initial washing step was included in the protocol because conservators routinely begin a treatment designed to reduce paper discoloration by bathing the object. To carry out this plan, seven samples of both the Whatman artists' paper (W56) and of the Whatman 1 paper (W1) were needed. One piece of each type of paper was reserved for analysis “as is.” Three pieces of each type were washed in trays of 0.4% (of saturation, by volume) Ca(OH)2 solution, and the remaining three pieces in 18.5 mM Mg(HCO3)2 solution (see appendix A for calculation of concentration). One of each group of three washed samples was reserved as the “washed only” sample. The remaining two samples in each group, after being dried and flattened, were reimmersed in trays of the bathing solution. While one of these was aqueously light bleached under a bank of daylight fluorescent lights, the other remained in the dark (the tray was wrapped in heavy-duty aluminum foil). Eight individual samples were treated simultaneously, four immersed in Ca(OH)2 solution (two W56 and two W1) and four immersed in Mg(HCO3)2 solution. Following this treatment, the bleached and dark-immersed samples were dried and flattened. Then every paper sample was cut in half. One half was artificially aged in a humid oven to obtain an indication of the long-term effectiveness of the different treatments. Procedural details are described in appendix A. A flow chart for the experimental procedure is shown in figure 1. The entire undertaking was repeated with different sheets of the two papers. The humid oven aging protocol was not reproduced exactly in the second experiment; details are given in appendix A. Statistical analyses of the data from each experiment were performed separately.
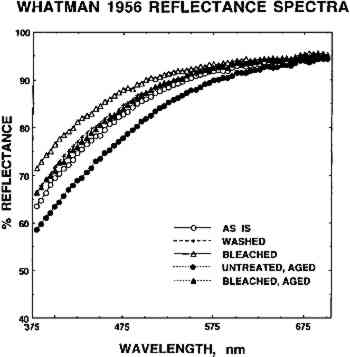
The effects of the various treatments on the papers were determined by objective assessment of the appearance, tensile properties, and some chemical properties of each sample. These properties were monitored after treatments and also after artificial, humid oven aging. The “as is” samples of each paper were analyzed before and after aging for comparative purposes. Appearance was assessed by reflectance spectrometry using a spectrocolorimeter. Reflectance spectra were recorded at four different places on each side of each sample. CIE L∗ a∗ b∗ values were computed by the instrument from the spectral data, and the readings were averaged. L∗ is an indication of the lightness or darkness of the sample, a∗ is a measure of the red-green value, and b∗ indicates the blueness or yellowness of the paper. The average values and population standard deviations are shown in tables 1 and 2. TABLE 1 WHATMAN 1 CIE L∗a∗b∗ COLOR VALUES TABLE 2 WHATMAN 1956 CIE L∗a∗b∗ COLOR VALUES Mechanical strength, stiffness, and brittleness of the papers were determined from features of the stress-strain curves obtained by slow elongation of 0.5 in. wide strips of each sample on a tensometer. All paper strips were mounted and stretched in the machine direction at a slow constant rate of elongation, until they broke. Four or five strips of each sample were measured, Two measures of chemical changes in the papers were used. First the pH of the paper samples was measured following the TAPPI cold extract pH method (TAPPI 1987b), but using commercial pH standards and one-half the weight of sample and volume of water called for in the published procedure. Gelatin content of the sized W56 samples was determined by a modification of the qualitative hydroxyproline assay for glue in paper published by Tappi (TAPPI 1987a). Purified photographic gelatin served as the standard for this semiquantitative procedure (Schaeffer 1995). Details of all the analytical methods are given in appendix B. 3 RESULTS3.1 SPECTROCOLORIMETRYExamples of reflectance spectra obtained for the sized artists' paper (W56) “as is,” after washing in Mg(HCO3)2 solution, and after aqueous light bleaching in Mg(HCO3)2 are shown in figure 2. The reflectances of untreated and of aqueously light bleached samples that had been artificially aged are also shown. The lighter in color a paper sample is, the larger percentage of the incident light that it reflects. Washing and aqueous light bleaching increased the reflectance of
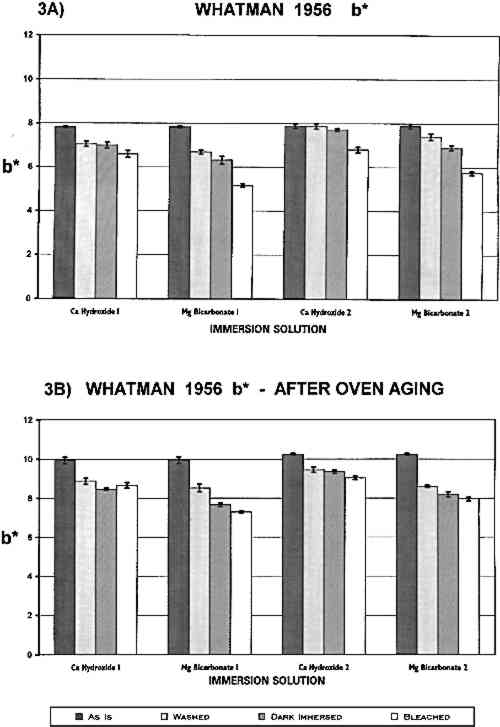
The complete colorimetric data are summarized in tables 1 and 2. It can be seen that the only treatments that caused significant changes in the unsized W1 paper (table 1) were aqueous light bleaching in the two immersion solutions, which reduced b∗ slightly. This finding may indicate that the W1 samples were less yellow after treatment, although the changes were too small to be detected by eye. (It should be borne in mind that the W1 paper looked “white” initially.) Ca(OH)2 and Mg(HCO3)2 were equally effective as immersion solutions. Humid oven aging increased the yellowness of all W1 samples (more positive b∗). The data suggest that in each experiment the samples that had been bleached in Ca(OH)2 solution may be slightly less yellow following artificial aging than the other W1 samples. Aqueous light bleaching in Ca(OH)2 and dark reimmersion in Mg(HCO3)2 made the gelatin-sized W56 paper appear slightly less yellow (table 2 and fig. 3). In both experiments, this paper was lightened somewhat more by aqueous light bleaching in Mg(HCO3)2 solution.
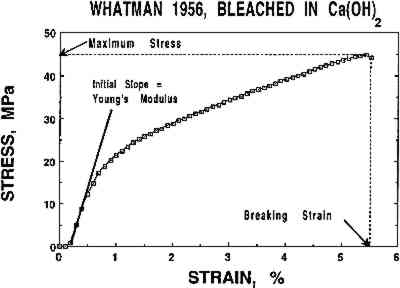
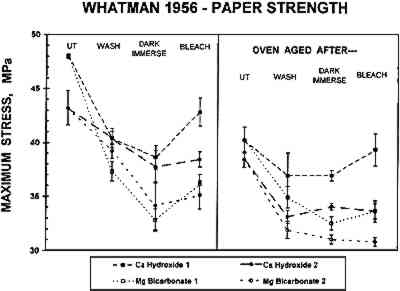
Humid oven aging consistently darkened all the W56 samples slightly (lower L∗; see table 2). In each experiment, these samples were yellowed significantly by the artificial aging process (increased b∗ in fig. 3b, cf. fig. 3a). The washed and bleached samples all remained less yellow than the untreated paper, and again in each experiment the samples that had been bleached in Mg(HCO3)2 appeared slightly less yellowed than those that were bleached in Ca(OH)2. However, it should be noted that the washed, dark reimmersed samples were not significantly more yellow after artificial aging than the corresponding bleached samples. 3.2 TENSOMETRYA plot of the stress (force per unit cross-sectional area) on a strip of paper as a function of the strain (percentage increase in length) in the paper while it is stretched to its breaking point can be used to determine several tensile properties of the paper. The three chosen for this investigation are maximum stress, which is an indicator of the strength of the paper sheet; the slope of the initial straight line portion of the curve (Young's modulus), which is a measure of the stiffness of the paper; and the breaking strain, which is inversely proportional to the brittleness of the paper and thus is a relative indication of its pliability. The stress-strain curve in figure 4 was obtained when a 0.5 in. wide strip of the W56 sample that had been washed and light-bleached in Ca(OH)2 solution was slowly stretched in the machine direction until it broke. The features described above are indicated on this graph. The same mechanical properties were evaluated for all samples. (The complete numerical data are available from the authors on request.)
The tensile properties of the untreated papers and the samples aged without other treatments are summarized in table 3. The gelatin-sized W56 paper was more than twice as strong and pliable as the unsized W1 paper, and Young's modulus of the W56 paper was almost twice as large as that for W1. Humid oven aging of the W1 paper did not change its tensile properties significantly. However, aging did reduce the strength and pliability and increase the stiffness of the W56 paper to some extent. Bathing each of the papers decreased the strength and stiffness somewhat and increased the pliability slightly. Changes in tensile properties caused by subsequent TABLE 3 TENSILE PROPERTIES OF UNTREATED PAPERS Differences in tensile properties of either paper due to the nature of the bathing solution were mostly insignificant or not reproducible. An exception to this behavior was the maximum strength of the W56 paper (fig. 5). In each experiment (square or circular symbols) samples bathed and reimmersed in Mg(HCO3)2 (open symbols) were significantly weaker than the samples that had been treated in Ca(OH)2 (solid symbols). These differences tended to persist upon artificial aging. Exposure to light did not appear to be a determining factor in this phenomenon.
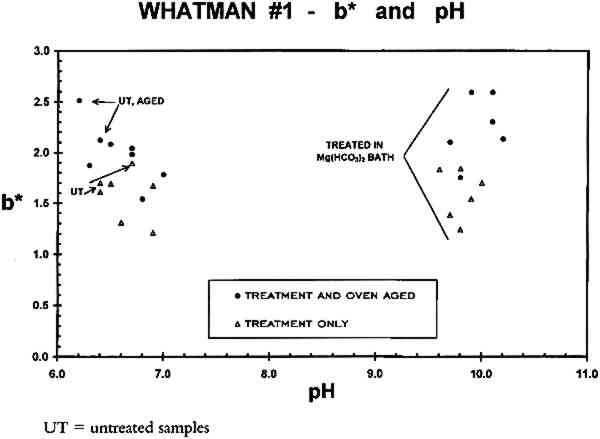
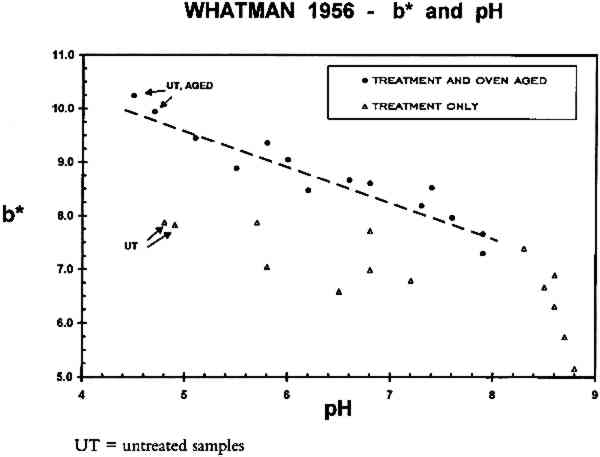
3.3 ACIDITYThe pH of the freshly diluted Ca(OH)2 solution (0.4% of saturation, or ca. 1.0 mM) was 10.1 � 0.2. This solution can neutralize acidic groups present at a concentration below 1 mM, but it cannot provide a buffer reserve once the neutralizing capacity has been used up. (The pH falls significantly as the solution sits in an open container, due to absorption of CO2 from the air. CaCO3, which has less than one-tenth the solubility of Ca(OH)2 in water, is formed and will precipitate out if the initial concentration of the hydroxide is too high.) Thus the Ca(OH)2 bathing solution is unlikely to provide a buffer reserve to acidic materials immersed in it. This effect can be seen in table 4 where the pH values, obtained by the cold extract method described in appendix B, have been listed. The procedure gives values accurate to ca. � 0.2 pH TABLE 4 COLD EXTRACT pH VALUES The Mg(HCO3)2 bathing solution is approximately 19 mM (see appendix A) and has a pH of ca. 7.0 initially. This solution has significant buffering capacity and imparts a buffer reserve to the sample papers, as can be seen from the pHs of the samples following immersion in this solution (table 4). In fact, bathing the acid-free W1 paper in Mg(HCO3)2 renders it distinctly alkaline. As a consequence, humid oven aging of these samples does not cause them to become acidic. The buffer reserve provided to the acidic W56 paper samples by two immersions in the Mg(HCO3)2 solution is also adequate to prevent these samples from becoming acidic as a consequence of artificial aging, although their pHs are lowered by about one unit. In both experiments, and for both the unsized and sized papers, the acidity of the aqueously light bleached samples is not significantly different from that of the samples that had been reimmersed in the dark. This finding was true both after initial treatment and after humid oven aging of the papers. The data indicate that exposure to light per se during immersion was without deleterious effect on this property of the paper samples. A correlation was observed between acidity and b∗ for the gelatin-sized, artificially aged paper samples, regardless of which treatment was used. This correlation is shown in figure 6, where b∗ of all W56 samples is plotted as a function of sample pH. Data from both experiments are included in this figure. The aged samples were in the humid oven for either 24 or 28 days (appendix A); it is not known whether the result would be the same for other aging times. The dashed line is the least-squares best fit (r = 0.947) to all the after-aging data points (solid circles). In contrast, there is no correlation between b∗ and paper acidity for the unsized W1 samples (fig. 7). The results of this investigation cannot supply a causal relationship for the observation, but the complete absence of a correlation for the unsized paper (fig. 7) suggests that the presence of gelatin sizing may be relevant. Barrett and Mosier (1995) recently reported correlations among lightness, pH, and gelatin content for a selected group of historic gelatin-sized papers.
3.4 GELATIN CONTENTImmersion of the heavily gelatin-sized W56 paper results in leaching of some gelatin sizing into the bath, as evidenced by the slight tendency of the solution to foam and to feel slick or sticky. To monitor this loss of sizing, the qualitative TAPPI test for glue in paper was modified for use as a semiquantitative assay (Schaeffer 1995). Some results of the application of this procedure are given in table 5, where data from at least four different determinations on samples from the two experiments have been averaged. Immersion of W56 samples in either Ca(OH)2 or Mg(HCO3)2 solution decreased the amount of gelatin in the paper, measured as percentage by weight of the analyzed sample. More gelatin was lost from the samples bathed in Mg(HCO3)2 than from those bathed in Ca(OH)2. The second immersion of the samples usually resulted in a further loss of gelatin, although not as much was lost as in the first bath. It should be noted that more than 40% of the gelatin sizing initially present in the untreated W56 paper had been removed after two immersions in the Mg(HCO3)2 solution. TABLE 5 PERCENT GELATIN BY WEIGHT OF WHATMAN 1956 SAMPLES 4 DISCUSSIONThe results presented here indicate that aqueous light bleaching in either Ca(OH)2 or Mg(HCO3)2 solutions, performed under conditions normally used by paper conservators in the laboratory, decreased the natural discoloration in both the unsized and the gelatin-sized cotton cellulose papers, usually to a greater extent than washing and dark immersion did. These papers were not markedly discolored initially, so the magnitudes of the bleaching effects were small. Although the decrease in yellowness was statistically significant in each experiment and detectable by eye for the sized paper, caution should be exercised in generalizing these results. Artificial, humid oven aging caused some color reversion in all samples. However, the samples that had undergone aqueous light bleaching were in no instance significantly darker than the washed samples. No deleterious change in paper appearance or mechanical properties could be attributed to light exposure itself, either immediately after treatment or after humid oven aging. A similar conclusion was reported in an earlier study that used a harsher bleaching process in the Weather-o-meter and dilute Ca(OH)2 as the immersion solution (Schaeffer et al. 1992). The relative efficacies of Ca(OH)2 and Mg(HCO3)2 immersion solutions for aqueous light bleaching to improve paper appearance are harder to evaluate. For example, no visible difference in appearance among any of the treated, unsized W1 paper samples was detected before or after aging. Although those samples bleached in Ca(OH)2 were measured to be slightly less yellow after humid oven aging, the differences in b∗ were too small to be discerned by eye. Many more repetitions of the entire experiment would be required to impart major significance to these data. The observation has been made that papers deacidified or bleached in Mg(HCO3)2 tend to appear more yellow than those bathed in dilute Ca(OH)2, especially upon aging (Hey 1979; Eldridge 1982), but that effect was not observed in this study. Rather, in both experiments, the W56 samples that were bleached in Mg(HCO3)2 appeared slightly less yellow than the samples bleached in Ca(OH)2. This result may be attributed, at least in part, to the presence initially of (slightly discolored) gelatin size and to removal of a larger amount of it by immersion of the paper in Mg(HCO3)2 than in Ca(OH)2. When the efficacy of aqueous light bleaching in the two immersion solutions is compared with respect to other paper properties, a different pattern emerges. The strength of the gelatin-sized paper is better maintained if it is washed and bleached in Ca(OH)2. The difference appears to be due to effects of immersion The stress values calculated in these experiments are inversely proportional to the thickness of the paper sample being tested. The thicknesses were measured with calipers (appendix B) and are thus nominal thicknesses. The washed papers had slightly increased thicknesses—ca. 6% for the unsized W1 paper and 15% for the W56 samples—increases that, even in the absence of loss of material from the papers, would lead to an apparent decrease in the calculated strength. However, the changes in the tensile properties of the treated papers were larger than could be accounted for by the thickness increases. The additional decreases in stress could be due to loss of soluble material that contributed to the paper strength and/or to chemical alterations of the components of the paper that shortened the fibers or decreased the fiber bonding. The present study was not designed to address this question. As expected, the effects of immersion on paper pH were different for the two solutions. Mg(HCO3)2 provided a good buffer reserve to the initially acidic gelatin-sized paper but caused the unsized W1 paper to become highly alkaline. In contrast, the dilute Ca(OH)2 solution did not provide to the W56 paper a buffer reserve that persisted upon humid oven aging. As a result, the aged W56 samples that had been immersed in Ca(OH)2 were distinctly acidic. Removal of significant amounts of gelatin size from the artists' paper by both immersion steps occurred throughout the investigation. This side effect of the conservation washing and deacidification procedure has been noted previously (Schaeffer et al. 1992; Schaeffer 1995). In the present experiments, significantly more gelatin remained in the W56 paper immersed twice in Ca(OH)2 than in Mg(HCO3)2. Because gelatin size contributes to the strength of the paper, the differences in the amount of gelatin lost to the immersion solutions could be partly responsible for the differences in the strengths of the treated samples. Paper conservators may want to consider the ramifications of immersion in Ca(OH)2 or Mg(HCO3)2 on the size content when they plan a treatment for a work on gelatin-sized paper. The sample papers used in this study were modern, new, or naturally aged papers made with 100% cotton α-cellulose fiber furnish. They did not contain lignin. There were no media or stains on them. Because of their content and condition, caution must be exercised when conclusions reached on the basis of these experiments are extended to stained materials, to papers with other sizing materials, to 19th- and 20th-century papers that may contain lignin, or to artworks on paper. The effects of treatment on the image materials or the chemicals composing the stain, and possible interactions among these substances and the components of the paper, all need to be considered. The effects of aqueous light bleaching on stained cotton cellulose papers (Schaeffer and Blyth-Hill 1993) are currently being investigated; findings will be reported separately. The results of the research presented here emphasize the fact that the side effects of a treatment procedure may be as important to the condition of the treated object as the primary result of the treatment. In the case of aqueous light bleaching, the choice of immersion solution—Ca(OH)2 or Mg(HCO3)2—may be influenced more by the pH, sizing, and strength of the paper than by the desired result of decreasing discoloration. As with other treatment considerations, the choice of immersion solution for aqueous light bleaching will need to be made on an individual basis. 5 CONCLUSIONS
ACKNOWLEDGEMENTSThe authors thank Dr. Glen Cass and Lynn Salmon of the California Institute of Technology for providing access to the Matchscan II spectrocolorimeter, Jack O'Neill of Sanofi Bio-Industries for the photographic grade gelatin, and Charles C. Hill for the Whatman 1956 artists' paper. Terry Trosper Schaeffer appreciates the hospitality of the GCI Science Section staff and thanks them for instruction in the use of the Instron tensometer and the computer graphics systems. John Twilley participated in many helpful discussions. REFERENCESBaker, C.1982. Practical methods for sun and artificial light bleaching paper. Book and Paper Group postprints, American Institute for Conservation 10th Annual Meeting, Milwaukee. Washington, D.C.: AIC. 14–15. Barrett, T., and C.Mosier. 1995. The role of gelatin in paper permanence. Journal of the American Institute for Conservation34:173–86. Branchick, T. J., K. M.Keyes, and F. C.Tahk. 1982. A study of bleaching of naturally aged paper by artificial and natural light. AIC preprints, American Institute for Conservation 10th Annual Meeting, Milwaukee. Washington, D.C.: AIC. 29–39. Bredereck, K., A.Haberditzl, and A.Bl�her. 1990. Paper deacidification in large workshops: Effectiveness and practicability. Restaurator11:165–78. Calvini, P., V.Grosso, M.Hey, L.Rossi, and L.Santucci. 1988. Deacidification of paper: A more fundamental approach. Paper Conservator12: 35–39. Eldridge, B. P.1982. A sun bleaching project. Book and Paper Group postprints, American Institute for Conservation 10th Annual Meeting, Milwaukee. Washington,D.C.: AIC. 1:52–55. Hey, M.1979. The washing and aqueous deacidification of paper. Paper Conservator4:66–80. Keyes, K. M., 1987. Alternatives to conventional methods of reducing discoloration in works of art on paper. In Conservation of library and archive materials and the graphic arts,ed.G.Petherbridge. London: Butterworths. 49–55. Lienardy, A., and P.vanDamme. 1989. R�sultats de recherches exp�rimentales sur le blanchiment du papier. Studies in Conservation34:123–36.
Lienardy, A., and P.vanDamme. 1990. Practical deacidification. Restaurator11:1–21. Schaeffer, T. T.1995. A semiquantitative assay, based on the TAPPI method, for monitoring changes in gelatin content of paper due to treatments. Journal of the American Institute for Conservation34:95–105. Schaeffer, T. T, M. T.Baker, V.Blyth-Hill, and D.van derReyden. 1992. Effects of aqueous light bleaching on the subsequent aging of paper. Journal of the American Institute for Conservation. 31:289–311. Schaeffer, T. T., and V.Blyth-Hill. 1993. Preparation of reproducibly stained paper samples for conservation research. Book and Paper Group annual. Washington, D.C.: American Institute for Conservation. 12:44–49. TAPPI. 1987a. Glue in paper (qualitative and quantitative methods). T 504 om-84. In TAPPI test methods 1988, vol. 1. Atlanta, Ga.: TAPPI Press. TAPPI. 1987b. Hydrogen ion concentration (pH) of paper extracts (cold extraction method). T 509 om-83. In TAPPI test methods 1988, vol. 1. Atlanta, Ga.: TAPPI Press. van derReyden, D., M.Mecklenburg, M.Baker, and M.Hamill. 1988. Update on current research into aqueous light bleaching at the Conservation Analytical Laboratory. Book and Paper Group annual, Washington, D.C.: American Institute for Conservation. 7:73–106. SOURCES OF MATERIALSPAPERWhatman 1, rectangular sheets 46 � 57 cmlocal laboratory supply house Whatman artists' paper, 1956private collection of C. C. Hill Waterleaf blotters, 112Paper Technologies, 929 Calle Negosio, Unit D, San Clemente, Calif. 92673 CHEMICALSPurified photographic gelatin, code. 41555Sanofi Bio-Industries, 620 Progress Ave., P.O. Box 1609, Waukesha, Wis. 53187 P-dimethylaminobenzaldehyde (Ehrlich's reagent)Sigma Chemical, P.O. Box 14508, St. Louis, Mo. 63178 N-propanol, spectrophotometric gradeAldrich Chemical, 1001 W. St. Paul Dr., Milwaukee, Wis. 53233 NaOH, CuSO4, H2SO4, and H2O2, all reagent grade, and MgCO3�Mg(OH)2�xH2O, USP gradelocal laboratory supply house INSTRUMENTS AND SUPPLIESLaboratory glassware, plastic ware, and bench top apparatuslocal laboratory supply house Vacuum filtration assemblyMillipore Corp., 397 Williams St., Marlborough, Mass. 01752, (other brands also available from laboratory supply houses) Fluorescent tubeslocal electrical supply house Gilson Pipetman 100 μl–1.00 ml automatic pipettorRainin Instrument Co., Mack Rd., P.O. Box 4026, Woburn, Mass. 01888 Japanese hole punchBookmakers, 6001 66th Ave., Ste. 101, Riverdale, Md. 20737 Orion Research, Inc., (available from local laboratory supply houses) Diano Matchscan II spectrocolorimeterBausch and Lomb, (this particular model is no longer available) Instron Automated Testing System tensometer, model 4201Instron Corp., 100 Royall St., Canton, Mass. 02021 JDC Model .5–10 precision sample cutterThwing-Albert Instrument Co., 10960-T Dutton Road, Philadelphia, Pa. 19154 Spectronic 20 spectrophotometerMilton-Roy Corp., 820 Linden Ave., Rochester, N.Y. 14626, (available via some laboratory supply houses) Temperature Humidity Chamber Model no. 435304Hotpack Corp., 10942 Dutton Rd., Philadelphia, Pa. 19154, APPENDIX1 APPENDIX1.1 DETAILS OF TREATMENT PROCEDURESCa(OH)2: Stock saturated calcium hydroxide solution was stored over solid material at room temperature in a closed container. This solution was filtered through Whatman 1 filter paper to remove undissolved material and immediately diluted into deionized water to a final concentration of 0.4% saturation by volume (4 ml filtered solution per 996 ml H2O). The pH of the diluted solution was 10.1 � 0.2 for different batches. Mg(HCO3)2: A stock magnesium bicarbonate solution is always kept on hand in the LACMA Paper Conservation Laboratory. It is prepared by suspending 200 g USP grade powdered MgCO3�Mg(OH)2�xH2O (approximately 42% as MgO) in 15 gal deionized water, letting the suspension stand overnight, and then bubbling CO2 through vigorously for 6 to 8 hours, by which time the solution is clear. This solution is diluted 1:1 (by volume) with deionized water immediately prior to use. Calculation of the concentration of the resulting Mg(HCO3)2 solution based on the percentage of MgO in the powdered material gives 18.5 mM. The pH of this solution immediately after the dilution has been made varies slightly from batch to batch of the stock material, in the range 6.8 to 7.2. Washing: Paper samples, ca. 7 � 21 in., were cut with the machine direction oriented in the long dimension of the piece. Samples to be washed were placed on a piece of Reemay and dampened with a fine spray of deionized water on both sides. Each piece was put into a 20 � 24 in. polyethylene tray containing 10 liters of the appropriate bathing solution and submerged gently with gloved hands. After 4 hours, the paper was removed and drained briefly on the Reemay, placed on waterleaf blotter, covered with another piece of Reemay and blotter, and blotted by carefully moving the hand over the top of the blotter. The Reemay sandwich was transferred to dry blotters and pressed under a sheet of Plexiglas and light weight for ca. 20 minutes. The washed papers were then transferred to fresh blotters and dried and flattened under Plexiglas and moderate weight for 3 days. They were stored between new blotters in the press until the next step of the experiment. Aqueous Light Bleaching and Dark Controls: Each washed piece of paper was cut into three pieces ca. 7 � 7 in. One of these was reserved as the washed-only control, and the other two were dampened and submerged on Reemay in individual 9 � 12 in. white polyethylene dissecting trays containing 1,500 ml freshly prepared Humid Oven Aging: Every paper sample was cut in half with a sharp scalpel, so that the machine direction of the halves was in their long dimension. Cotton thread, 150 gauge, was sewn into the corners of one-half piece of each sample. These half samples were individually tied into Plexiglas frames with the sheet surface oriented in the vertical plane and the machine direction vertical. All four corners of each sample were tied so that the paper pieces could not swing around or come in contact with each other. In the first experiment, the samples were aged in the dark at 70 � 0.5�C and 50 � 4% relative humidity for 28 days in a Hotpack model 435304 temperature-humidity chamber. (The aging protocol was not duplicated precisely in the second experiment; the humidity fluctuated well beyond the limits used before, and the process was terminated after 24 days. The data for the two experiments have not been combined in the statistical analyses.) At the end of the aging period, the oven was turned off and the door left open until the Plexiglas frames did not feel hot to the touch. The frames with the samples tied into them were placed in a cupboard in the dark for ca. 3 hours to cool. The samples were then cut out of the frames and stored in polyester photograph sleeves. 2 B. ANALYTICAL PROCEDURESColorimetry: The appearance of all samples was monitored by colorimetry. Reflectance spectra from 380 to 700 nm were measured at 2 nm intervals on a Diano Matchscan II instrument with a white tile as the reference material and another white tile in back of the paper samples. This second tile was also used to calibrate the instrument before each use. Four scans at different positions on each side of each sample were recorded using a 2� observer and the small (.25 in.) aperture. Specular reflection was excluded. CIE 1976 L∗ a∗ b∗ values were computed for a D65 illuminant by the instrument from the data of each scan. The values for the four scans of each side of each sample were averaged. If recto and verso values were not significantly different, the average of all eight scans is reported; otherwise recto values are given. All average values are given � population standard deviation. Tensile Behavior: Tensile properties of the samples were determined by evaluation of stress-strain data obtained with an Instron 4201 instrument using Series IX Automated Materials Testing System. The sample papers were equilibrated in the laboratory at least 24 hours before they were tested. Strips of paper precisely 0.5 in. wide were cut from each sample using a JDC Model .5–10 precision sample cutter. The pH: The acidity of the paper samples was measured by the cold extraction method for pH, TAPPI test method T 509 om-83, slightly modified as follows. Because of the limited amount of sample material available, one-half the weight of paper and one-half the volume of solution specified in the procedure were used for each determination. The sample was cut into small pieces, and 0.50 � 0.05 g weighed on an analytical balance. The sample was placed in a stainless steel Waring blender minicontainer. Then 37.5 � 0.5 ml deionized H2O were added, and the material was blended for 10 seconds. The sides of the container were scraped down, and the slurry blended for a further 10–20 seconds, depending on the extent of breakdown of the paper sheet during the first blending period. The slurry was transferred to a clean 150 ml beaker, covered with Parafilm, and allowed to stand 1 hour. The pH of the suspension was measured with an Orion Ross-style model 8135 flat surface combination electrode and a Markson model M-6071 pH meter, after N2 had been bubbled through the slurry for 10 minutes. This procedure generally provides pH values accurate to � 0.2 pH units. The electrode was calibrated with commercial standard buffers of pH 7.0 and 4.0 before use each day and was checked for drift every few hours. This type of electrode is particularly stable, drifting ≤ 0.03 pH 7.0 and 4.0 before use each day and washing solutions and those used for reimmersion and aqueous light bleaching were also measured, both before and after their use, with the same electrode. GELATIN ASSAY: The amount of gelatin in the Whatman 1956 samples was determined by a semiquantitative modification of TAPPI standard procedure T 504 om-84 for qualitative determination of glue in paper (Schaeffer 1995). Briefly, ca. 4-12 mg of paper sample was extracted in 0.5 ml 5 M NaOH at 100�C. The extract with the paper samples still in it was analyzed for hydroxyproline using Ehrlich's reagent, and the absorbance of the resulting colored solution was read on a Milton-Roy Spectronic 20 spectrophotometer. the amount of gelatin present was determined by comparison of the absorbance with a standard curve generated for each assay, using purified photographic grade gelatin (Sanofi Bio-Industries). The modified method has been shown to give results that are good to � 10%, which was adequate for comparing the relative amounts of gelatin sizing remaining in the samples after each of the different treatment steps. AUTHOR INFORMATIONTERRY TROSPER SCHAEFFER received a Ph.D. in biophysics from the University of California at Berkeley in 1967. After more than 20 years of research in photosynthesis and membrance transport, and occasional university teaching, she entered the field of conservation science in 1989–90. Since that time she has studied the effects of aqueous light bleaching on paper and devised laboratory procedures to facilitate research projects in this area. Her interests include the accelerated aging of paper of experimental purposes and the effects of conservation treatments on modern and historic papers. Address: Los Angeles County Museum of Art, 5905 Wilshire Blvd., Los Angeles, Calif. 90036. VICTORIA BLYTH-HILL is the senior paper conservator in the Conservation Center for the Los Angeles Country Museum of Art. She is past chair of the Book and Paper Group of the AIC and has been a Fellow of that organization since 1988. She has presented lectures at AIC and other professional meetings and at universities on subjects ranging from general conservation awareness to the treatment of the Leonardo da Vinci Codex Hammer and a large-scale decoupage by Henri Matisse. Blyth-Hill has supervised many interns at LACMA and has sponsored several externally funded research and survey projects. She also organized the Western Association of Art Conservation symposium, “Conservation Treatment of Tibetan Thankas.” Address: Los Angeles Country Museum of Art, 5905 Wilshire Blvd., Los Angeles, Calif. 90036 JAMES R. DRUZIK has been a conservation scientist at the Getty Conservation Institute since 1985. His principal research interests concentrate on environmental chemistry and paper conservation, and he is responsible for administering GCI's contract research activities. In 1988, 1990, 1992, and 1994 he, along with Pamela Vandiver, Edward Sayre, George Wheeler, and Ian Freestone, organized and conducted a series of international symposia entitled “Materials Issues in Art and Archaeology” for the Materials Research Society. Prior to joining GCI, he worked in the Conservation Center, LACMA, under both Victoria Blyth-Hill and Pieter Meyers from 1980 to 1985, and from 1970 to 1980 at the pasadena Museum of Modern Art and the Norton Simon Museum. Druzik has a B.S. in chemistry. Address: Getty Conservation Institute, 1200 Getty Center Dr., Ste, 700, Los Angeles, Calif. 90049–1684.
 Section Index Section Index |