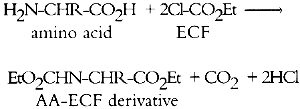GAS CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS AS ETHYL CHLOROFORMATE DERIVATIVES.MICHAEL R. SCHILLING, HERANT P. KHANJIAN, & LUIZ A. C. SOUZA
1 INTRODUCTIONProteins are an important class of natural organic materials that have long been used as binding media and adhesives. Of the many proteins found in nature, the ones encountered most frequently in conservation include those of glue (obtained by boiling animal hides and bones), size (a more purified form of glue), casein (present in milk), glair (egg white), and egg yolk (K�hn 1986). In essence, proteins are biopolymers of amino acids. Amino acids have the general formula H2N-CHR-CO2H, where R is a functional group that imparts unique chemical and physical properties. Proteins may be broken down into their constituent amino acids by the action of strong aqueous acid. Either liquid acid or acid vapor may be used for this purpose (Pickering and Newton 1990). This process, known as hydrolysis, is an important first step in many of the analytical methods used for identification of proteins. Twenty natural amino acids are commonly found in protein hydrolysates, and many others have been identified (see appendix 1). One way that proteins differ from each other is in their relative proportions of amino acids. For example, glue is characterized by high levels of hydroxyproline, glycine, and alanine, while casein contains large amounts of aspartic acid and glutamic acid but no hydroxyproline. Amino acid composition is often used as the basis for identification of proteins. A number of analytical methods can be used to identify proteins directly, or through their acid hydrolysates, including spot tests (Collings and Young 1976; Stulik and Florsheim 1992); thin-layer chromatography (Masschelein-Kleiner 1974; Striegel 1994); high-performance liquid chromatography (HPLC) (Sinkai and Sugisita 1990; Halpine 1992; Grzywacz 1994; Ronca 1994); and ion chromatography (Keck and Peters 1969; Sack et al. 1981; Pickering 1989). Gas chromatography (GC) is an analytical technique that has been applied to study all the major classes of organic binding media. Overviews of the general procedures may be found in Mills and White (1978, 1987) and in Pancella and Bart (1989). These methods have A number of GC procedures have been developed for identification of proteins. Before GC analysis, amino acids in protein hydrolysates must first be converted to volatile derivatives; this conversion requires reaction of both the amine and the carboxylic acid functional groups. Various authors have taken different approaches to derivatization, yielding results that have not been completely satisfactory (Masschelein-Kleiner 1976; White 1984; Pancella and Bart 1989). The main difficulty with all existing GC methods reported in the conservation literature is excessive sample preparation time. Derivatization may require from 4 to 24 hours, involving many tedious chemical reactions with the potential for analyte losses at each transfer step. Clearly, a need exists for a rapid derivatization method that is suitable for analysis of protein present in art objects and artifacts. In a procedure for analyzing acid hydrolysates of blood serum specimens, Hušek (1991) used ethyl chloroformate (ECF) to form N(O,S)-ethoxycarbonyl amino acid ethyl esters via the following reaction:
Utilizing split injection of a chloroform solution of the derivatives onto a capillary GC column coated with OV-1701 cyanopropyl stationary phase for separation, total derivatization and analysis time was 4 minutes. Nineteen of the common amino acids were quantified by this method, with the exception of arginine, which irreversibly adsorbs onto the column because the imino group on the guanidine moiety fails to derivatize. Nonetheless, the technique (hereafter referred to as the ECF method) satisfied the criterion of minimal sample preparation and analysis time and warranted investigation for possible uses in conservation. Because proteins are identified by comparing amino acid compositions of unknown samples to known materials, it is important to have access to a source of amino acid data for commonly encountered proteinaceous substances. Proteins associated with art objects, artifacts, and monuments may come from various sources. As mentioned earlier, binding media and adhesives are major uses of proteinaceous materials. Besides glue, casein, and egg, other materials have traditionally been used as binding media and adhesives that may contain small amounts of amino acids or proteins, such as wheat starch, rice starch, and blood (Sinkai and Sugisita 1990). Other organic media that are not normally considered proteinaceous may contain small but measurable quantities of amino acids. For instance, certain plant gums are composed of glycoproteins (Sharon 1975). Samples from objects may contain amino acids that originate from ubiquitous proteinaceous contaminants. Handling of objects leads to transfer of small but measurable quantities of amino acids present in skin, such as alanine, valine, leucine, glycine, proline, aspartic acid, threonine, serine, lysine, and histidine (Hamilton 1965). Outdoor objects and historic buildings may be exposed to microbes (Ronca 1994), guano, and other proteinaceous environmental contaminants. The cleaning action of saliva on painted objects is directly attributable to the presence of enzymes; after treatment, the enzymes may remain on the painted surface (Rom�o et al. 1990). Over time the contamination of objects with proteinaceous substances is a virtual certainty, resulting in confusing amino acid compositions. In general, it is difficult to locate amino acid concentration data on the wide variety of proteins that are associated with the conservation of works of art. Even the excellent compilation by Mills and White (1987) lacks information on proteinaceous contaminants. Thus, an |