A HISTORY OF PEST CONTROL MEASURES IN THE ANTHROPOLOGY COLLECTIONS, NATIONAL MUSEUM OF NATURAL HISTORY, SMITHSONIAN INSTITUTIONLISA GOLDBERG
ABSTRACT—Since the mid-19th century, various pest eradication techniques have been employed on the anthropology collections at the National Museum of Natural History, Smithsonian Institution. These techniques are reviewed, and pesticide and fumigant use by early collectors and later collections management staff is documented. Also chronicled are the ways in which the choice of chemicals has changed over the years and the decisions that led to those changes. The effects of pest eradication techniques on the collections are discussed, and the author's findings are offered as the basis for further research. 1 INTRODUCTIONHistorical information concerning the use of pesticides and fumigants is important to understanding and evaluating the care and treatment museum collections have received in the past. An account of pest control measures for anthropology collections at the National Museum of Natural History (NMNH), Smithsonian Institution, illustrates many trends in collections management during the 19th and 20th centuries. Pesticides and fumigants have long been used in various departments at the National Museum of Natural History to control and prevent damage from insects and rodents. At different times, departments preferred the use of specific chemicals. Poisons and pest control choices were dictated by the type of collection (botany specimens, for example, were treated differently from vertebrate zoology collections) and by concern for efficacy and human health and safety. Interest in human safety and health hazards associated with pesticide use has long been of concern to natural scientists and museum professionals. In the 18th century the hazards of mercury compounds were recognized by Pierre-Jean-Claude Mauduyt: “Corrosive sublimate [mercuric chloride] is a dreadful poison, which should be entrusted only to an artist…. To place it into ignorant or reckless hands is to entrust them with a weapon with which by nearly touching it they can injure themselves” (quoted in Farber 1977, 556). Pesticide use in museum collections has evolved from the application of a wide array of chemical compounds to the development of specific pest management procedures that recognize the effects of pesticide use on personnel and collections. Currently, the use of many fumigants and pesticides requires specific licensing and, in the United States, registration with the Environmental Protection Agency. For details regarding the properties and status of chemicals discussed in this article, see table 1. TABLE 1 SUMMARY OF PESTICIDE AND FUMIGANT PROPERTIES Pest control measures for anthropology collections have included an assortment of choices over the years as perceptions about the need for gross collections maintenance and individual object treatment changed. In the late 19th and early 20th centuries, collections management staff at NMNH were referred to as “museum aides” or “museum preparators.” By the 1940s individuals caring for collections worked in the anthropology laboratory. In the 1960s, a separate Anthropology Conservation Laboratory (ACL) was created for restoration and preservation, and the Processing Laboratory Documentation of chemical use for anthropology collections has not been chronicled by specific object or collection group because such treatments were traditionally thought to be part of general collections maintenance. However, a survey of the use of individual pesticides over the years can indicate which collections were most likely treated with specific pesticides or fumigants. This information can then aid in evaluating past object treatments, determining the potential presence of residues, and evaluating the stability of various ethnographic materials. 2 HISTORY OF PEST CONTROL IN ANTHROPOLOGY COLLECTIONS, NMNH2.1 SOURCESThe following history of the use of pesticides in the anthropology collections at NMNH was reconstructed from written and verbal sources. Museum and expedition records from the mid-19th century suggest that pest control was carried out by museum staff and collectors using both heat and chemicals. Pest eradication in the museum environment for the latter part of the 19th century and the first two decades of the 20th century is documented in several early publications. Documents in the Smithsonian Institution Archives (SIA) also contain information on the use of poisons. In 1940, a lengthy memorandum was sent to the associate director of the National Museum (precursor to NMNH) to document changes in the types of treatments that accompanied the advent of closed storage cabinets; it served as a manual for the treatment of insect infestations (Manual 1940). Prior to 1955, a few collections records include treatments for specific types of materials or objects, recipes for poisonous concoctions, and examples of treatments involving small collections, specific items, and their cases. From 1965 to 1973, the Smithsonian published general pest control reports that documented the use of specific chemicals by individual departments within the institution, which are filed in the SIA. Information about the recent use of pesticides was obtained from archival records and from conversations with staff members in various departments at NMNH. Current information is from collections management records in the Anthropology Department. 2.2 LATE 19TH- AND EARLY 20TH-CENTURY USE OF PESTICIDES AND FUMIGANTSIn the second half of the 19th century and the early years of the 20th century, collections were treated by both field collectors and museum personnel. Early evidence suggests that 19th-century collectors such as Captain Charles Wilkes regularly applied poison to their anthropological and biological specimens during collecting trips to ensure en route survival. Other collectors used the materials they had at hand. On June 18, 1893, Voth described his field preservation efforts as follows: “I have thrown tobacco among the articles and hope that it will keep until the collections can be unpacked in Washington” (SI, acc. record no. 26674, NMNH). Field collectors regularly employed a wide range of chemicals to reduce pest damage in transit. Documents in SIA indicate that “fumigating tobacco,” camphor, “flour of sulphur,” arsenic, and “corrosive sublimate” (mercuric chloride) were purchased for field collecting use to aid in the preservation of specimens. Papers from the National Institute (precursor to the National Museum) indicate purchase of these chemicals for both the U.S. Exploring Expedition and for individuals working in Washington on collections received. These records date from the mid-1830s through the The care of collections received from expeditions was rarely well documented. Collectors such as Captain Charles Wilkes left general instructions for specimen care upon arrival. For example, Wilkes wrote to Paulding on November 9, 1840, “that in order to keep them in a state of preservation … it is of the highest importance, that they be moved as little as possible, and kept unopened, in a safe and dry depository” (quoted in Poesch 1961, 72). But some archival evidence suggests that collections maintenance did not always meet the standards of the expedition itself. Charles Pickering wrote to T. R. Peale that “when the boxes and packages were placed in charge of the National Institute, the seals were broken, and a general scramble for the curiosities took place … many valuable specimens were lost—especially shells and skins of birds” (SI, RU 7186, page 23). Collections care in the museum (the National Institute) caused further problems for continued specimen classification and research. On May 15, 1844, T. R. Peale wrote to Professor Frazer, “I cannot forget the late exploring expedition—my two birds (male and female) made into one … arrows in another with their ends sawed off to make them fit into fancy stands, etc.” (quoted in Poesch 1961, 96). Although there are few specific references to the use of poisons on unpacked specimens, purchase records indicate that John Varden (curator of the U.S. Exploring Expedition and for the National Institute) procured quantities of arsenic, camphor, “fumigating tobacco,” and occasional “corrosive sublimate” for the “further care and preservation of specimens brought home by the U.S. Exploring Expedition” (SIA, RU 7058, series 4, box 14). Varden may have used heat as a method of pest eradication as chronicled in his diary of museum work, dated 1843–65 (SIA, RU 7063). For example, on Monday, August 1, 1859, he recorded that he was “at work Baking a fine collection of Indian Dresses collected by Lt. G. Warren U.S.I. which I found in bad condition and the chest in bad order which was repaired for the collection” (SIA, RU 7063). Throughout his diary there are other references to the use of heat on Indian “curiosities,” skins, and birds. While Varden did not specifically say that he “baked” these items to free them of pests, a Smithsonian report entitled “Methods of Preserving Lepidoptera” describes the use of an oven, invented in 1828, for the eradication of “dermestides populations” (Peale 1864). In 1868, Smithsonian Institution expenses included a “heating room for collections” (Rhees 1901, 675). References to the use of heat as a drying agent for preservation and pest eradication of natural history specimens can be found from the late 18th century through the 19th century and are especially prevalent in descriptions of ornithological collections (Lettsom 1774; Stearns 1952; Farber 1977). In the latter part of the 19th century, the Smithsonian Institution sponsored collection expeditions by supplying equipment and expertise (Rhees 1901). Field collectors were accompanied by museum preparators, but the distinction between their roles is sometimes difficult to ascertain. For example, records indicate that Mr. Palmer treated objects with a mixture of arsenic and mercury compounds, labeling these objects with tags that read “Palmer: poisoned” (Manual 1940). Palmer may have been a museum preparator, but as a Arsenic and mercury compounds had long been used by natural scientists who valued their preservative properties for natural history specimens. In Smithsonian period publications, arsenic was recommended as an essential supply for collectors (Baird 1854). Comments in the 1940 memorandum suggest that specimens treated with arsenic were very rarely subject to new infestation problems. In 1887, Dr. Walter Hough, a museum copyist and head curator of the Anthropology Department, described a variety of methods and mixtures for pest eradication of anthropological items, including recipes for poisonous concoctions and suggestions for pest management. Hough recommended the following as a general insecticide for museum objects: 1 pt. saturated solution of arsenic acid and alcohol, 25 drops strong carbolic acid, 20 grains strychnine, 1 qt. strong alcohol, and 1 pt. naphtha, crude or refined. He found this solution “most satisfactory for poisoning nearly every kind of specimen…. The use of strychnine is not absolutely necessary; but it is a very good agent and adds much to the value of the solution” (Hough 1889, 553). Hough (1889) recommended that application vary with the type of object: fragile objects were sprayed with an atomizer, while small specimens were dipped and larger items were painted. He also suggested the manufacture of an arsenical powder or paste for objects that could not be sprayed, using burnt alum and powdered oak bark, or precipitated chalk, powdered soap, or vaseline, as media. Three types of arsenic compounds were mentioned by Hough and other scientists of the period, including “arsenic acid,” “white arsenic” or arsenic trioxide, and “arseniate of potash.” Water-soluble arsenic acid, or orthoarsenic acid, was probably used in most liquid formulations. Arsenic trioxide was the most common compound used in the preparation of dry arsenical soaps and powders. The addition of “sol soda” (potassium or sodium bicarbonate), potash, saltpeter, or other hydroxides to arsenic trioxide would form a salt, allowing dissolution into water or alcohol and the formation of something akin to sodium or potassium arsenite. “Arseniate of potash” may have referred to potassium arsenate, or “Macquer's salt,” a compound that was used in insecticidal formulations. A few collections records that probably date prior to 1940 report the use of arsenic. These include recipes for arsenical baths: “Mix a handful of sol soda, a quart or two of water and 1 pound of arsenic. Boil until dissolved. Add 30 gallons of water/alcohol (1:1). Specimens should soak for 2 days to a week” (ACL collections records). A second recipe on the same page varies slightly: “� pound of arsenic is added to 2 gallons of water. Boil and add an equal part of alcohol. This mixture is used as a paint for fresh skins, sides of robes, feather garments and the insides of bird skins” (ACL collections records). There are no records indicating when arsenic ceased to be used on anthropology collections, but arsenic is still used in the field preparation of vertebrate zoology specimens (Jacobs 1993). Hough did not retire from the Anthropology Department until 1935, and it is possible that he continued to treat the collections he was responsible for with the same mixtures throughout his tenure. Although the end date for arsenic use on collections is not known, the storage unit doors were fitted with felts that had been pretreated with arsenic. These arsenic impregnated felt strips were added to all wooden storage cabinets to create a pest-free seal. The use of sodium arsenate on storage door felts was reported in 1959, and its use until the mid-1980s was confirmed by the manufacturers (Pest Control 1959; McClaine 1990). Felt strips were treated with arsenic until recently, when the museum's supply of arsenic was depleted (Angle 1990). Mercuric chloride (corrosive sublimate) was Objects were either dipped in or were painted with the mercuric solution. Early collection records indicate that closed drawers of objects were protected by scattering crystalline mercuric chloride in the corners and over particularly vulnerable objects such as textiles. The 1940 manual confirms this use, and Mr. Allen may have performed this duty until his departure in the 1930s. Hough recommended the use of poison
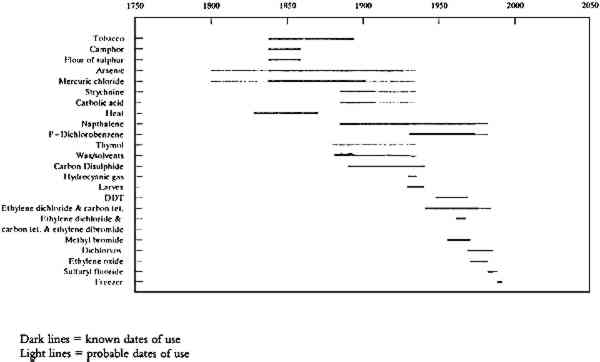
Naphthalene was also recommended by Hough (1889) as a preventive measure against potential moth infestations. He suggested sprinkling crystals on objects or putting “cones” of this material in drawers (Hough 1889, 556). Naphthalene “cones” were constructed by piercing chunks of the crystalline solid with a pin (Riley 1892). Paradichlorobenzene was first mentioned as a fumigant solution for exhibit cases in 1931, but in the years that followed, both naphthalene and paradichlorobenzene were used interchangeably (Krieger 1931, 10). For closed cases, these chemicals were used as volatile preservatives in addition to other pesticides and fumigants. This chemical usage is confirmed by entries in federal pest control reports for the Anthropology Department dating from 1969 to 1973 (SIA, RU 155, box 130; SIA, RU 157, box 31; SIA, RU 197, box 43). Both chemicals were referred to as moth flakes or mothballs, although the words “flakes” and “balls” usually indicate naphthalene, while “crystals,” “nuggets,” and “cakes” generally signify paradichorobenzene. Naphthalene was also used by Hough in conjunction with menthol or thymol (5-methyl-2-isopropyl 1-phenol) as another moth preventative (Hough 1889). He also advised the use of a mixture of alcohol, salicylic acid, thymol, and perfume of lemon for “domestic purposes” (Hough 1889), but it is not clear how this solution was applied. It is also unclear if these solutions were used on specimens in the anthropology collections. Although thymol does not seem to appear in later references and sources as a fumigant of choice, it was used by various departments as a fungicide. Early collections records also indicate that several types of objects were treated in solvent baths, followed by impregnation with wax, in an attempt to protect them against insect attack. This treatment was described in articles by Hough, Krieger, and others as a method of removing the oils and resins to which museum pests are commonly attracted. Hough also recommended that objects should be soaked with benzene prior to the use of other poisons. Types of objects treated in this manner include wooden objects, baskets, and feathers. Collections records did not mention this procedure as a preventive treatment for skins or furred items, but wax-solvent mixtures may have been used on these items to induce flexibility. The dates for these treatments are unknown, but the style of these records indicates that they occurred prior to 1940 (ACL collections records). 2.3 20TH-CENTURY USE OF AGRICULTURAL PESTICIDES AND GASEOUS FUMIGANTSIn 1913, with the advent of closed storage cabinets, the use of volatile poisons became more prevalent (Manual 1940). In the following years, liquid and powder treatments with arsenical and mercuric compounds decreased as museum professionals relied on volatile compounds such as carbon disulfide and, later, paradichlorobenzene, ethylene dichloride–carbon tetrachloride, and others. Carbon disulfide was noted as long in use by museum professionals in 1931 (Leechman 1931), and its use in NMNH probably began before 1889 (Hornaday 1891). In the 1940 manual, carbon disulfide was mentioned as in use, but its expense and potential for explosion made it less attractive than other volatile fumigants. Hough described an airtight tank for “carbon bisulphide” (carbon disulfide) fumigation, but he recommended that treated specimens be soaked in an arsenic-naphtha solution afterward (Hough 1889). According to undated early 20th-century collections records, carbon disulfide was allowed to vaporize in sealed storage units over the course of several days. Presumably, this chemical was poured into a bowl or container that was then locked inside a storage unit. Carbon disulfide was considered an explosive hazard, and it is unclear how long it remained in use. Hydrocyanic acid gas is also mentioned as a fumigant for rugs (Krieger 1931). This treatment is not mentioned in the collections records, but Krieger indicated that private companies provided facilities for this treatment. In the 1930s, preparators also experimented with the use of Larvex (sodium aluminum fluorosilicate) and Entol (?) for treatment of large incoming collections such as the Evans Collection (Manual 1940). Collections records suggest that the beaded skin costumes and feather headdresses in the Evans Collection were sprayed with these products, but other large groups of objects that arrived during this time may also have been treated with the same chemical. In 1946, DDT (dichloro-diphenyl- Concurrently, collections management staff in the Anthropology Department maintained pest-free storage areas by treating the collections with volatile mixtures of chlorinated hydrocarbons, such as ethylene dichloride and carbon tetrachloride. These chemicals were not mentioned in Krieger's report, suggesting that their use postdates 1931. In 1940, an ethylene dichloride and carbon tetrachloride (3:1) mixture was cited as a choice fumigant for semi-annual, in situ fumigation, despite concurrent use of carbon disulfide (Manual 1940). The use of door portals as vehicles for gaseous fumigant delivery dates to 1962, as described in the museum's annual report: “A liquid fumigant is now being placed in tin cups inside of the storage doors and is renewed semi-annually … the insecticide used is a mixture of carbon tetrachloride, ethylene dichloride and ethylene dibromide [Dowfume G]” (Kellogg 1962). Dowfume G was used from 1961 to 1967, as recorded in a memorandum from Mr. Michaels of November 1961, and in the Federal Commission on Pest Control report for calendar year 1967 (SIA, RU 155, box 74). Dimethyl formamide was also poured into these tin cups, as reported in an estimate for yearly use (65 gallons) for 1965 (SIA, RU 155, box 74). However, staff most commonly recollect the use of Dowfume (presumably Dowfume 75), containing ethylene dichloride (70%) and carbon tetrachloride (30%). Most of the old storage unit doors are fitted with tags marked “poisoned” and bearing stamps for nearly every year from 1961 to 1977, indicating a fumigation sequence with chlorinated hydrocarbons or dimethyl formamide. Chlorinated hydrocarbon products probably included Dowfume G and Dowfume 75. In situ fumigation of storage cabinets continued for at least another decade as a spot treatment for units with observed pest activity (Brown 1990; Eisenhart 1990; Wilcox 1990). Use of Dowfume G and Dowfume 75 was gradually abandoned within the museum in response to concerns over health and safety of staff members in close proximity to fumigated storage cases (SIA RU 155, box 74; SIA RU 197, box 28). In 1970 and 1971, these issues generated two studies of air quality both inside and outside storage units to ascertain Dowfume 75/85 levels during pesticide treatments From 1942 through 1955, collections records indicate that collections with noted infestations were taken to the “fumatorium” (fumigation chamber) for treatment. All new accessions and susceptible collections were treated in the fumatorium between 1942 and 1949 (Setzler 1943, 1944, 1946, 1949). One undated collections record noted that the fumatorium was used for fumigation with a mixture of ethylene dichloride and carbon tetrachloride (ACL collections records). Later archival records suggested that a mixture of ethylene dibromide, ethylene dichloride, and carbon tetrachloride was also used within this room. Four different Dowfume products are mentioned, including Dowfume 75, Dowfume 85, Dowfume G, and Dowflor, in documents found in “Pest Control” files (SIA). Staff recollections established that all incoming collections were fumigated in this chamber prior to 1970 (Elder 1990). In 1969, the fumatorium was closed because of questions about its use and operation. Evidence for closure of the facility was collected by James E. Mabry of the U. S. Department of Agriculture, and an institution-wide memorandum from the Secretary of the Smithsonian Institution was circulated later in the year (SIA, RU 155, box 130). There are several different accounts of chemical use in this room. Verbal reports suggest that a liquid chemical was pumped from a tank in front of a fan and was allowed to circulate (Brown 1990; Elder 1990). A memorandum dated June 4, 1969, from Mabry and a document by David Lellinger of the Botany Department describe transfer via an open container of a mixture of ethylene dichloride (70%) and carbon tetrachloride (30%) from a 55 gal. steel drum to a metal fumigation chamber inside the fumatorium (SIA, RU 155, box 130). Archival records also indicate that there was some confusion about the actual chemical within the fumatorium. The administration expressed concern about the health hazards relating to the use of brominated compounds, while the scientific staff referred to their use of chlorinated compounds (SIA, RU 155, box 130). The Vertebrate Zoology and other natural science departments seem to have recorded only the use of Dowfume 75. Although the Anthropology Department used brominated Dowfume G from 1961 to 1967, its use may have been confined to in situ fumigation of storage cases. Memorandums and other correspondence referred to the use of Dowfume 85, or 85% liquid ethylene dibromide, but there is no evidence that it was used. None of the departmental reports to the Federal Commission on Pest Control recounted use of this specific product. Verbal sources alluded to the use of methyl bromide in the National Museum of Natural History from about 1957 until 1971 (Brown 1990; Elder 1990; Greenwell 1990), although archival references cite its use only during the latter part of this period. Several individuals remembered use of this chemical in the fumatorium because of its red color and distinctive odor. Methyl bromide only exists as a gas at normal atmospheric pressure, so these references probably describe another brominated compound, perhaps ethylene dibromide (Dowfume 85). Methyl bromide was used by the Botany Department in a sealed pressure chamber under vacuum, off site at the USDA (SIA, RU 155, box 130; Lellinger 1990). Use of the USDA facilities and equipment for fumigation occurred between 1969 and 1971 while the fumatorium was nonfunctional (SIA, RU 197, box 28). After the closure of the fumatorium in 1969, archival records indicate active institutional interest in the use of Dichlorvos (2,2 - dichlorovinyl dimethyl phosphate, also known as DDVP or Vapona) as an alternative to gaseous fumigation within NMNH. The Smithsonian Institution and Shell Corporation, manufacturer of Vapona, jointly sponsored The Anthropology Department participated in research pertaining to the use of Dichlorvos, as reported in the Federal Commission on Pest Control reports for 1969 and 1973 (SIA, RU 99, box 332; SIA, RU 197, box 43). These reports contain references to the use of “No-Pest Strips” (DDVP-impregnated polyvinyl chloride resin strips) in an attempt to find a safer solution for in situ fumigation. In the late 1970s and early 1980s, staff also experimented with a limited number of commercially available plastic tags containing a reservoir of DDVP. These were placed inside closed storage units, but the treatment was considered unsuccessful (Eisenhart 1990; Valentour 1990; Wilcox 1990). No-Pest Strips or Vapona strips were also sporadically placed in storage units and exhibit cases with evidence of pest activity (Brown 1990; Eisenhart 1990; Valentour 1990). By 1983, Dichlorvos was no longer in use for storage cabinet fumigation because of reports of chemical odors emanating from treated storage units (Wilcox 1993). These strips and tags are still occasionally found in storage drawers, and one example postdates DDVP use to 1985. In response to concern about the volatility of various Dowfume products and the need for chemicals that acted faster than Dichlorvos, the Botany Department installed a hospital mattress pad sterilizer in the fumatorium for use with ethylene oxide gas. The Botany Department was responsible for the operation of the fumatorium and this fumigation unit from 1971 until 1983, when use of this unit was discontinued due to concerns about its potential for malfunction as well as in response to new Occupational Safety and Health Administration (OSHA) standards and provisions for operation (Makos 1990, 1995; *Russell 1990, 1995). This fumigation unit sterilized specimens with Carboxide, a mixture of ethylene oxide (90%) and carbon dioxide (10%), under vacuum, at elevated temperatures. The unit was meant to “sterilize at 95–110�F,” and the maximum temperature was 135–40�F (SIA, RU 137, box 330). There was at least one record of a temperature malfunction in which a set of botanical specimens were subjected to a temperature much higher than normally expected (SIA, RU 257, box 9). Botany Department personnel fumigated anthropology collections with ethylene oxide as needed. Use of this chemical by the Anthropology Department may have fluctuated widely; a summary of “fumigator” use for fiscal year 1972 indicates that the Anthropology Department used the facility only four times, but accession records for individual objects in the Processing Laboratory indicate much more frequent usage (SIA, RU 257, box 9). Routine fumigation was not a policy; only collections or individual items with suspected or active pest activity were fumigated in this chamber (Russell 1990; Phebus 1993; Wilcox 1993). Often individual objects were fumigated with specimens from other departments such as Botany (Wilcox 1990). By 1983, fumigation within NMNH was no longer considered feasible. Objects with active or suspect pest problems were transported to outside contractors for treatment with Vikane (sulfuryl fluoride). Two different companies were used (Western Pest Control Co. until 1986 and Paramount Exterminating Co. after that), and lists of treated objects were kept by the collections management staff. Records indicate that selected groups of items were treated in this manner until 1986, but staff recollections suggest that use of this fumigant continued until 1989 (Eisenhart 1990). 2.4 USE OF NONCHEMICAL PEST CONTROL MEASURESAs concerns about collections care focused more narrowly on collections with active pest infestations, maintenance treatment emphasized the isolation and inspection of suspect items. As early as 1949, weekly inspections of exhibits were undertaken “to forestall any serious infestation” (Setzler 1949, 16). In the 1970s some incoming collections were isolated and monitored to ascertain whether live pests were present (Eisenhart 1993; Norman 1993). By the early 1980s collections were vacuum-cleaned to rid them of all pest debris before restorage. During the latter part of the 1980s and the early 1990s, collections were inspected and cleaned when necessary to remove evidence of previous pest activity. During this time, individual storage drawers were also fitted with sticky traps to monitor suspected pest activity on an intermittent basis. This shift from mass treatment to pest management limited chemical exposure and reduced the need for object treatment. In 1989, the Anthropology Conservation Laboratory purchased a freezer for pest eradication of individually infested items. This freezer is used in a treatment schedule recommended to kill insect life in all phases of its life cycle (Florian 1986). To date, a variety of infested or potentially infested objects have been successfully frozen and rendered pest free. Low-temperature treatment is used only when inspection and vacuum-cleaning reveal the presence of an active or recent pest problem. Records for this treatment are kept by the Anthropology Conservation Laboratory. As collections have been moved to the Smithsonian's new Museum Support Center in Suitland, Maryland, pest management has adhered to the philosophy of preventive conservation. Collections are now housed in new metal cabinets that are closed and gasketed. The entire storage area is regularly monitored, in keeping with the facility's integrated pest management plan. 3 HEALTH AND SAFETYThe preceding survey of pesticide and fumigant use for anthropology collections at the Smithsonian uncovers an array of potential toxins. Table 1 summarizes exposure limits and health effects for each of these specific chemicals. Museum personnel concerned with exposure potential for previously applied pesticides should evaluate each chemical that may have been used in terms of time and method of application, volatility, and other physical characteristics. For example, metal salts such as arsenic or mercury could exist as crystalline residues, while carbon disulfide would probably not leave any gaseous traces. Residues for many chemicals may be traced using specific chemical tests. Consultation with an industrial hygienist can help in an evaluation of potential exposure. With so little information on actual exposure levels in the museum workplace, individuals caring for historically poisoned collections should take precautions necessary to limit exposure (Peltz and Rossol 1983). Many previously used pesticides and fumigants could potentially have long-term health effects when there is inadvertent exposure. While the medical effects of exposure to many of these chemicals have been described in terms of standard threshold exposure limits (STEL) or time-weighted averages for an 8-hour period (TWA), there is scant research on exposure levels for museum professionals who handle treated materials but do not apply these chemicals themselves. Early research on pesticide residues in museum collections confirmed the presence of arsenic and mercury on objects, but results concerning dermal and inhalation exposure were inconclusive due to small sample size and variance in work habits (Muir et al. 1981). Similarly, test results for worker exposure to DDT residues in museums showed that concentrations were well below governmental standards (Aviso 1985). More recent work at the Smithsonian Institution confirmed that nonvolatile pesticide residues 4 EFFECTS OF PESTICIDE & FUMIGANT TREATMENTS ON COLLECTIONSLittle is known about the potential changes that can occur to objects treated with individual or multiple pesticide or fumigant treatments. Much of the available research concerns the effects of these chemicals on individual materials under relatively short-term conditions. More recent research on the effects of pesticides and fumigants on museum collections has indicated that these chemicals can cause a wide variety of changes in metals, waxes, resins, oils, pigments, dyes, cellulosic, and protein-based materials (Dawson 1988; Baker et al. 1990). The anthropological collections under consideration here were treated over a long period of time. They are often constructed from diverse or multiple materials. For these collections, observed changes resulting from pesticide and fumigant treatments are often not immediately visible due to visual evidence of wear, use, or manufacture and previous conservation efforts. Many items exhibit stains, tide lines, or areas of white, crystalline bloom, and a determination of their source must be considered on an individual basis. Many of these collections exhibit relatively little evidence of serious pest damage, indicating that past pest management techniques were successful. Any changes in the materials themselves must be counterbalanced by the recognition 5 CONCLUSIONSIn the early years of the Smithsonian Institution, pest control measures were carried out by collectors and museum preparators. During the first half of the 20th century, the responsibility for pesticide and fumigant treatments shifted from collectors to collections maintenance staff. As concern about human safety became more prevalent, the focus of pest control changed from the treatment of entire collections to the treatment of items with active infestations. The paucity of records regarding the use of pesticides on specific items within the anthropology collections has required reconstruction of a very general history of the use of specific chemicals. Early published reports, archival records, collections notes, and conversations with various museum personnel have helped to delineate the uses of these chemicals to specific time periods. General dates for the uses of specific pesticides and fumigants are summarized in figure 3. This time frame can be used to help determine which collections might have been treated with particular pesticides at the time of their arrival in the museum.
Furthermore, this history of collections management practices for anthropology collections provides some general information about the treatment of these vast collections. Recent re-evaluations of the effects and efficacy of pesticide and fumigant treatments have led to a more complete understanding of the interactions among museum personnel, collections, and these chemicals (Dawson 1988). Many institutions with natural history collections have similar histories of pesticide and fumigant use. The historical survey presented here provides a basis for further investigations into the potential health hazards for individuals entrusted with their care. ACKNOWLEDGEMENTSI would like to thank Mary Ballard, Candace Greene, Greta Hansen, Stephen Koob, and Dennis Piechota for their encouragement in this research. I would also like to thank all the individuals whom I interviewed for their recollections. Catherine Hawks and Jane Walsh were generous in providing me with direction and reference suggestions. The staff at the Smithsonian Institution Archives were all extremely helpful in my pursuit of evidence. Thanks are also due to Kathryn Makos of the Smithsonian's Office of Environmental Management and Safety for helping to provide information included in table 1 and to Catharine Zwiesler for graphing the data in figure 3. Lastly, I would like to thank Lori Schlenker and Barbara Watanabe for their help and support. NOTES. Dust samples were collected from objects on dampened cotton swabs. Mercury was tested for by wetting out a dust sample with a 0.01% solution of diphenyl-carbazone. Arsenic was tested for by wetting out a dust sample with deionized water, followed by the addition of zinc dust, cuprous chloride, and conc. hydrochloric acid. A commercial version of this test is available through Merck Scientific. As with most spot microchemical tests, there is great margin for false results (Feigl et al. 1972, 111, 307–8). REFERENCESAngle, P. 1990 and 1992. Personal communications. Collections Manager (1964–present), Birds Division, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560.
Anthropology Conservation Laboratory. Collections records. Anthropology Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. Aviso. 1985. Museums can be hazardous to your health. Aviso June 1–2. Baird, S. F.. 1854. Directions for collecting, preserving and transporting specimens of natural history. Prepared for the use of the Smithsonian Institution. Washington, D.C.: Government Printing Office. Baker, M. T., H. D.Burgess, N. E.Binnie, M. R.Derrick, and J. R.Druzik. 1990. Laboratory investigation of the fumigant Vikane. ICOM Committee for Conservation preprints, 9th Triennial Meeting, Dresden. Paris: ICOM. 2:804–11. Baxter, D., and G.Gottfried. 1992. Evaluation of residual preservatives in selected natural history collections. Task order 36. Office of Environmental Management and Safety, Smithsonian Institution, Washington, D.C. Brown, J.1990. Personal communication. Museum Specialist (1960–87), Processing Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. CFR Federal Register 53 FR 15977 May 1988. Washington, D.C.: National Archives and Records Administration. Dawson, J.1988. The effects on insecticides on museum artifacts and materials. In A guide to museum pest control, ed.L. A.Zycherman and J. R.Scrock. Washington, D.C.: Foundation of the American Institute for Conservation of Historic and Artistic Works and the Association of Systematic Collections. 135–50. Elder, R. A.1990. Personal communication. Museum Specialist (1941–83), Processing Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. Eisenhart, L.1990 and 1993. Personal communications. Collections Manager and Museum Specialist (1975–91), Anthropology Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. EPA. 1990. Suspended, cancelled, and restricted pesticides. Pesticide and toxic substances (ENC-342), 20T-1002. Washington, D.C.: Environmental Protection Agency. Farber, P. L.1977. The development of taxidermy and the history of ornithology. Isis68:550–66. Feigl, F., V.Anger, and R.Oesper. 1972. Spot tests in inorganic analysis. New York: Elsevier Publishing Co. Florian, M.-L. E.1986. The freezing process: Effects on insects and artifact materials. Leather Conservation News3:1–13, 17. Greenwell, F.1990. Personal communication. Museum Specialist (1963–92), Vertebrate Zoology Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. Hawks, C. A., and D. W.Von Endt. 1990. Mercury and mercury compounds in natural history collections: An annotated bibliography. Natural History Conservation5:4–19. Hawks, C. A., and S. L.Williams. 1986. Arsenic in natural history collections. Leather Conservation News2.2:1–4. Hornaday, W.1891. Taxidermy and zoological collecting. New York: Charles Scribner's Sons. Hough, W. A. M. 1889. The preservation of museum specimens from insects and the effects of dampness. In Annual report of the board of regents of the Smithsonian Institution for the year ending June 30, 1887. Washington, D.C.: Government Printing Office. Pt.2:549–58.
Jacobs, J. O.1993. Personal communication. Museum Specialist (1984–present),Vertebrate Zoology Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. Kellogg, R.1962. Report of the United States National Museum. Washington, D.C.: Smithsonian Institution. Krieger, H. W.1931. Care and preservation of museum specimens. Museum News8:9–11 Leechman, D.1931. Technical methods in the preservation of anthropological museum specimens. In Bulletin 67: Annual report for 1929. Ottawa: Canada Department of Mines, National Museum of Canada. 127–58. Lellinger, D.1990. Personal communication. Curator (1963–present), Botany Department. National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. Lettsom, J. C.1774. The naturalist's and traveler's companion, containing instructions for collecting and preserving objects of natural history and for promoting inquiries after human knowledge in general. London: E. and C. Dilly. Makos, K.1994 and 1995. Personal communications. Senior Industrial Hygenist (1988–present), Office of Environmental Health and Safety, Smithsonian Institution, Washington, D.C. 20560. Manual on insect infestations and their treatments. 1940. Memorandum to Mr. Graf, via H.S. Barber, Anthropology Conservation Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. Mason, O. T.1902. Aboriginal American basketry: Studies in a textile art without machinery. In United States National Museum Annual Report, 1902. Washington, D.C.: Government Printing Office. 171–548. McClaine, C.1990. Personal communication. Cavetown Planing and Mill, Cavetown, Md. Merck and Co. 1983. The Merck index. 10th ed.Rahway, N.J.: Merck and Co. Muir, D., M.Lovell, and C. P.Peace. 1981. Health hazards in natural history museum work. Museum Journal80(4):205–6. Norman, J.1993. Personal communication. Conservator (1973–85), Anthropology Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. Office of the Federal Register. 1991. Code of federal regulations. 29, pt. 1910. Rev. July 1, 1991. Washington, D.C.: Office of the Federal Register. OSHA. 1991. OSHA Instruction CPL, 2-2.43A. Washington, D.C.: Occupational Safety and Health Administration. Peale, T. R.1864. Method of preserving lepidoptera. In Annual report of the board of regents of the Smithsonian Institution, 1864. Washington, D.C.: Government Printing Office. 404–6. Peltz, P., and M.Rossol. 1983. Safe pest control procedures for museum collections. Conservation hazard and data sheet 8. New York: Center for Safety in the Arts. Pest Control. 1959. Pest control in galleries and natural history museums. Pest Control27(11):9–12. Phebus, G.1990 and 1993. Personal communications. Collections Manager (1965–83), Processing Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. Poesch, J.1961. Titian Ramsey Peale, 1799–1885, and his journals of the Wilkes Expedition. Philadelphia: American Philosophical Society. Processing Laboratory. Records. Anthropology Department, National Museum of Natural History, Washington, D.C.: Smithsonian Institution.
Rhees, W. J.1901. The Smithsonian Institution: Documents relative to its origins and history, 1835–99. Washington, D.C.: Government Printing Office. Riley, C. V.1892. Directions for collecting and preserving insects. United States National Museum Bulletin39(F):1–147. Washington, D.C.: Government Printing Office. Russell, R.1990, 1993, and 1995. Personal communications. Collections Manager (1975–present), Botany Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. Sax, N. I., et al. 1984. Dangerous properties of industrial materials. 6th ed.New York: Van Nostrand Reinhold Co. Setzler, F.1943. Department of anthropology. In Report of the United States National Museum, 1943. Washington, D.C.: Government Printing Office. 17–27, 210. Setzler, F., 1944. Department of anthropology. In Report of the United States National Museum, 1944. Washington, D.C.: Government Printing Office. 16–25. Setzler, F.1946. Department of anthropology. In Report of the United States National Museum, 1946. Washington, D.C.: Government Printing Office. 16–28. Setzler, F.1949. Department of anthropology. In Report of the United States National Museum, 1949. Washington, D.C.: Government Printing Office. 5–21. Smithsonian Institution Accession Record Number 26674. National Museum of Natural History, Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 99. Office of the Secretary records. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 137. Under Secretary records. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 155. Office of the Director, National Museum of Natural History records. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 157. Buildings Management Division Records. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 197. Office of the Director, National Museum of Natural History records. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 257. Office of the Director, National Museum of Natural History records. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 7058. National Institute records. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 7063, John Varden papers. Smithsonian Institution, Washington, D.C. Smithsonian Institution Archives. Record Unit 7186, United States Exploring Expedition Records. Smithsonian Institution, Washington, D.C. Stearns, R.1952. James Petiver: Seventeenth century promoter of natural science. Proceedings of the American Antiquarian Society62(2):363–65, app. 2. Valentour, C.1990. Personal communication. Conservator (1976–87), Anthropology Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560.
Wilcox, V.1990 and 1993. Personal communications. Collections Manager (1975–91), Anthropology Department, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560. AUTHOR INFORMATIONLISA GOLDBERG graduated from Mount Holyoke College with a B.A. in art history. She completed her conservation training at the Institute of Fine Arts, New York University, with a diploma in art conservation and an M.A. in art history. She worked at the National Museum of Natural History, Smithsonian Institution, Washington, D.C. as move conservator for the Anthropology Department from 1990 to 1994. She is presently in private practice. Address: 401 Belford Place, Takoma Park, Md. 20912.
 Section Index Section Index |