INVESTIGATION OF A SURFACE TARNISH FOUND ON 19TH-CENTURY DAGUERREOTYPESLEE ANN DAFFNER, DAN KUSHEL, & JOHN M. MESSINGER
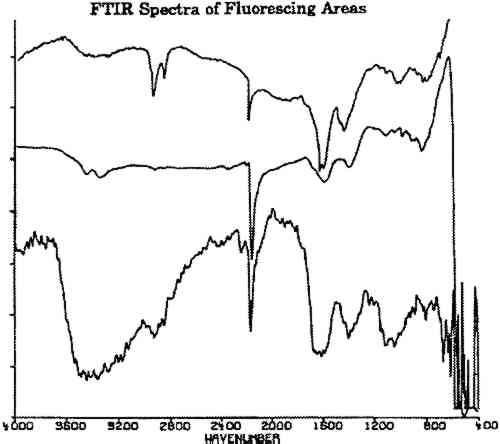
7 FOURIER TRANSFORM INFRARED SPECTROSCOPYFourier transform infrared spectroscopy (FTIR) yields data on organic and inorganic compounds present on a sample. For analysis, a bare daguerreotype plate was simply placed on the platform of the infrared microscope. No contact was made between the microscope and the plate, avoiding damage to the surface. The three spectra shown in figure 6 were obtained on the fluorescing areas of two daguerreotypes (figs. 2, 4) using a Nicolet 710 spectrometer equipped with an IR-PLAN infrared microscope. The uppermost spectrum in figure 6 was taken from a fluorescing edge. The two lower spectra in figure 6 were taken from two concentric rings on the daguerreotype in figure 4, one of the daguerreotype plates that was used for the SEM analysis. The spectrometer was operated in the reflectance mode using a 15� cassegrainian infrared objective, at 8 cm−1 resolution.
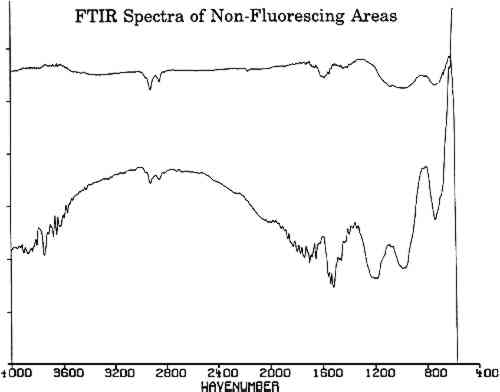
All three spectra of figure 6 show a strong absorption band at 2165 cm−1, which is not present in spectra of adjacent, nonfluorescing areas (fig. 7). This absorption band is within the wave number range in which the cyanide ion and the carbon-nitrogen triple bond bands groups absorb. Since there are no other common organic groups showing a strong absorption in this region other than a carbon-nitrogen triple bond, one plausible explanation is that the observed absorption is a cyanide compound. The spectra of the fluorescent deposits indicate that the residues are mixtures, composed of what appears to be a cyanide compound and the salt of an organic acid. The infrared analysis cannot identify the specific cation associated with the cyanide ion. The cyanide absorbances, however, are consistent with the absorbances expected for either potassium or gold cyanide (Bellamy 1968). Elemental analysis techniques such as micro x-ray diffraction might be able to identify the material if it is crystalline. If the crystals are too fine, however, this technique will not provide an adequate diffraction pattern for positive identification.
|