THE U.S. FIRST LADIES GOWNS: A BIOCHEMICAL STUDY OF SILK PRESERVATIONMARY A. BECKER, POLLY WILLMAN, & NOREEN C. TUROSS
ABSTRACT—The degradative changes in silk are investigated with amino acid analysis and solubility under denaturing conditions (7M urea). These biochemical techniques use extremely small samples for quantitative analyses and are capable of detecting chemical alterations at the molecular level. The biochemical results are used to place the fabric samples from a museum collection within the context of artificially aged silks. Analyses of the naturally aged samples reveal two populations: sericin-rich and sericin-depleted. Denaturing solvents such as soap will remove the protective protein coating from sericin-rich fabrics; sericin-depleted fabrics are already a high risk for light-induced damage. The data suggest that silk fabrics manufactured during this century are likely to have their sericin coatings totally removed and therefore at great risk to light damage. 1 INTRODUCTIONIn 1987, the exhibition of the U.S. First Ladies gowns, a popular and important Smithsonian Institution collection, closed for renovation to allow the curatorial staff to reassess the collection's use and to give conservators a chance for long overdue examinations and treatments. This conservation project provided a unique opportunity for extensive collaborative research between scientists and conservators into the deterioration of silk, the material predominant in this collection. The scientific research focused on, first, the effectiveness of several analytical approaches with minimal destructive sampling as a means of evaluating the object's state of preservation, and, second, the mechanisms involved in the degradation of silk. Since silk is a biopolymer, the effects of the environment on both the physical and the chemical properties over time are of interest to biochemists and bio-engineers as well as conservation scientists. Traditional textile testing methods such as measuring color difference and tensile strength have a limited usefulness in evaluating naturally aged fabrics with unknown histories. Although color measurements can be made nondestructively, a color difference cannot be quantified because the original color is unknown. Tensile strength measurements require large samples by museum standards and then provide evidence of deterioration only after significant damage has occurred at the molecular level. Previously, biochemical techniques were used to quantify the initial degradative changes found in artificially aged silk fabrics. Quantitative results can be obtained from very small samples. Becker and Tuross (1994) reported that the selective degradation mechanism progressed to random destruction along the molecular polymer chain with increasing length of exposure and increasing energy content of the incident radiation. This paper discusses how biochemical investigations are used to relate naturally aged silk fabrics from a museum collection to artificially aged silk fabrics. The changes monitored in solubility and amino acid compositions are indicators of deterioration at the molecular level. The overall recovery of amino acids not only provides evidence of the extent of protein preservation but also serves as an indication of the state of preservation of the garment. A comparison between the overall amino acid composition of naturally aged silk and that of new, degummed silk (essentially pure fibroin), provides insight into the presence or 2 HISTORY OF THE FIRST LADIES COLLECTIONThe collection of costumes and accessories from the First Ladies of the United States of America is part of the Division of Political History at the Smithsonian Institution's National Museum of American History (NMAH), Washington, D.C. Fifteen silk gowns attributed to the First Ladies of the White House were displayed as part of a Period Costumes exhibit in the original U.S. National Museum building (now known as the Arts and Industries Building), opened to the public on February 1, 1914 (Smithsonian Institution 1914). The term “First Lady,” first used in 1860, is given to the “hostess” of the White House. Within just a few years, the exhibit was declared “one of the most interesting and popular in the Museum” (Smithsonian Institution 1919, 66). Of the original 15 silk gowns on display at the opening of the hall in 1914, 12 were still on display when the exhibition hall closed in 1987, and 11 had been shown continually during this 73-year period. The exhibit was initially located in the west-north range of the Arts and Industries Building (A&I), where the gowns were exposed to sunlight through large, west-facing windows, although these windows were intermittently protected by heavy curtains. In 1931 the exhibition was transferred to the north-west range of A&I where they could be illuminated with north light in order to make “artificial lighting of the collection unnecessary” (Smithsonian Institution 1931, 12) (see fig. 1). Daylight was not eliminated from the Collection of Dresses of the First Ladies of the White House exhibit until the construction of a series of period-room displays in the 1950s. Reduction of artificial light levels was initiated during the installation of the First Ladies Hall in the new Museum of History and Technology building (now NMAH) in 1964. Currently light levels in areas displaying the First Ladies costumes are 3 footcandles or less.
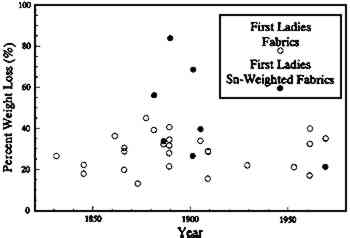
Over the years the gowns in the First Ladies Collection have been subjected to various forms of museum and user handling as well as different types of light. The collection is accompanied by irregular historical documentation on the garments, covers a broad time period from 1780 to 1989, and consists of fabrics in variable states of preservation. Of the 44 gowns on exhibit in 1987, 42 are constructed primarily of silk. The deleterious effects of light and dirt, as well as the demands of long exhibition, are so serious that some of the gowns are now too fragile to be exhibited. The silk fabrics in the gowns display a variety of museum-acquired degradative states. One example, Sarah Yorke Jackson's wedding dress, crumbling at the hem, was in such poor condition that now only the embroidered chiffon skirt is exhibited as part of a conservation section of the reopened exhibition. On the other hand, the silk in Barbara Bush's gown is in pristine condition. 3 SILK: FIBROIN AND SERICINSilk fibroins (Howitt 1946; Lucas et al. 1958; Rudall 1960) are the extracellular proteinaceous filaments produced by certain species of the phylum Arthropoda, classes Insecta and Arachnida. Many species belonging to the class Insecta spin cocoons in which they pupate, such as the classic example, Bombyx mori (order Lepidoptera, subclass Bombycidae). This domesticated silkworm, actually a caterpillar, spins its cocoon after feeding on mulberry leaves for nearly a month. The cocoon consists of two extruded triangular-shaped filaments of a proteinaceous polymer called fibroin, held together by a gumlike protein called sericin. Removal of the sericin from silk fibroin is accomplished by a process called “degumming,” usually by one of three methods: (1) extraction A fundamental distinction between fibroin and sericin is the composition of amino acids that make up these two proteins (see table 1). Characteristic features of the fibroins in general are the high proportion of the smaller side group amino acids glycine, alanine, and serine and their insolubility in aqueous solutions. The large percentage of the small side-chain amino acids allows for a close-packing arrangement of the molecules characteristic of the fibroins and is a general feature of extracellular matrix proteins. Sericin, on the other hand, has over 30 mol % serine (Komatsu 1980) and is readily soluble in hot, dilute alkali solutions (Lucas et al. 1960). TABLE 1 AMINO ACID COMPOSITIONS OF BOMBYX MORI SILK PROTEINS (mol %) 4 MATERIALS4.1 FABRICS FROM THE FIRST LADIES COLLECTIONFabric samples were taken from several of the gowns that had been on exhibit in the First Ladies Hall when it was closed for renovation in 1987 (see table 2). Whenever possible, examples of silk fabric were taken from TABLE 2 FIRST LADIES GOWNS SAMPLED 4.2 ARTIFICIALLY AGED SILK HABUTAEA plain woven silk habutae (Testfabrics style #609) was used for comparison to the naturally aged fabrics in the First Ladies Collection. The use of commercial degumming, causing removal of the sericin, was confirmed by amino acid analysis of the material extracted (0.5% of fabric weight) after boiling the habutae in deionized water for 30 minutes (table 1). The hot water extract had an amino acid composition similar to the amorphous region of silk fibroin, not sericin, confirming that the fabric was completely degummed (Becker 1993). The new silk fabric was artificially aged by exposure to a xenon arc light source in an Atlas Ci-65 Weather-ometer (Atlas Electric Devices 1986). Fabric samples were mounted in sample holders with four exposed areas of 42 � 50 mm per holder and irradiated for varying lengths of Light (3.8 hours) and dark (1.0 hours) exposures were alternated to simulate the radiative environment that occurs during the lifetime of museum objects. The average operating temperature in the Weather-ometer was 21�C, and the relative humidity fluctuated between 55% and 63% with the cycling. 5 METHODS5.1 SOLUBILITYNew silk fibroin goes into solution only with very aggressive treatments, such as those using LiBr or LiI, which degrade the molecule, rendering it partially or totally soluble (Lucas et al. 1958). Urea was chosen as the solvent for this study because of its known denaturing properties (Stryer 1988) that allow the protein molecules to assume a random-coil chain conformation with no evidence of main chain peptide cleavage. All samples were extracted in 1 ml of 7 M urea, 0.05 Tris(tris(hydroxymethyl) aminomethane) solution (pH 8) for one week with constant rocking and occasional vortex mixing to facilitate dispersion of the solution throughout the fabric. After one week, the urea-Tris solution was removed, and the remaining fabric was rinsed a minimum of five times with deionized water. The fabric samples were prefrozen, then lyophilized to dryness. Percent weight loss was calculated using the comparison of preextraction to postextraction dry weights. 5.2 AMINO ACID ANALYSISMicrogram-sized fabric samples were weighed and put into hydrolysis tubes, dissolved in 200–400 μl of 6N HCl, flushed with nitrogen, and tightly capped. The samples were then hydrolyzed at 110�C for 22 hours. After hydrolysis, samples were dried in a vacuum evaporator at 50�C and stored dry at −20�C until just prior to chromatographic separation. All samples from the First Ladies gowns were filtered through 0.45 μm Millipore filters to remove particulates prior to injection. Asparagine and glutamine residues are deamidated to aspartic and glutamic acid upon acid hydrolysis (Robinson and Rudd 1974) and therefore reported as the sum of both the acid and amide (Asx and Glx). Amino acid compositions of 45 silk fabric samples from the First Ladies gowns were determined using reverse-phase high-pressure liquid chromatography (RP-HPLC) on a Millipore Waters Pico-Tag Amino Acid Analysis system. Amino acids were precolumn derivatized with phenylisothiocyanate (PITC) and separated by RP-HPLC (Bidlingmeyer et al. 1984; Cohen et al. 1986). Amino acids were eluted using a gradient buffer system and detected by UV absorbance at 254 nm. Amino acid compositions of the artificially aged silk fabrics were determined by ion-exchange high-pressure liquid chromatography (IE-HPLC) (Benson and Hare 1975; Stathoplos 1989). Primary amino acids were separated by ion-exchange and detected by postcolumn derivatization with o-phthaldialdehyde /2-mercaptoethanol (OPA) to form fluorescent-substituted isoindole products. Two runs were required to adequately separate the basic amino acids from the interfering buffer peaks (Hare 1977). For analysis, hydrolyzed samples were diluted to a concentration of 1 μg/μl. The compositions are based on daily calibrations to standard solutions of each amino acid reported. 5.3 DESALTING THE PROTEIN-UREA EXTRACTIONSUrea was removed from the silk that came into solution using a rapid desalting procedure (Cooper 1977). Rapid desalt columns were prepared from a slurry of polyacrylamide gel (Bio-Gel P-6DG) and 50 mM ammonium acetate (pH 7.2) loaded into 10 ml disposable pipettes. A column was calibrated for a 2 mL sample with bromphenol blue (670 MW) and blue dextran (2,000,000 MW) dissolved in urea. Samples were loaded onto the individual columns, eluted with 50 mM ammonium acetate, and collected into vials. Sample collection was terminated just prior to the elution volume of bromphenol blue, before the urea eluted. Samples were lyophilized, resuspended in distilled water, and then relyophilized to obtain a final dry weight. 6 RESULTS AND DISCUSSION6.1 SOLUBILITY DATAMore than 88% of the silk fabrics evaluated from the First Ladies costumes lost more than 20% of their dry weight under denaturing conditions (fig. 2). Half the naturally aged fabrics had weight losses of greater than 30%. Although a 7M urea solution is more aggressive than a traditional conservation treatment with a mild detergent, it illustrates the strong possibility for material loss with solvent cleaning.
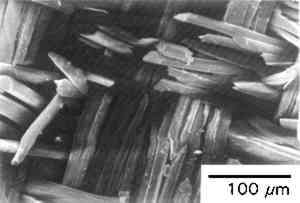
There is also a variation in the amount of weight loss in urea between different fabrics from the same garment. American-made silk fabrics were used for both Caroline Harrison's and Mary Harrison McKee's gowns, dating to President Benjamin Harrison's 1889 inauguration. In the 7M urea, fabrics from Caroline Harrison's gown had a 32–84% weight loss, while fabrics from Mary McKee's gown exhibited a 22–35% weight loss. Dating to a similar period, Frances Cleveland's 1886 wedding dress lost 32% and 34% of its original weight for the satin and lining fabrics respec- tively. These high percentages of weight loss cannot simply be attributed to removal of dust or other particulate matter that has adhered to the fabrics but is indicative at least in part of the loss of actual silk protein. The fabric samples known to be tin weighted tend to be more soluble than many nonweighted silk fabrics. Tin used as a weighting agent has been reported by conservators to accelerate the deterioration of silk based primarily on the loss in tensile strength of the fabrics (Bogle 1979). Caroline Harrison's apricot faille (1889) exhibited an 84% weight loss after urea extraction. This faille was also particularly friable when handled physically. Julia Grant, Edith Roosevelt, Ida McKinley, and Frances Cleveland's gowns contained tin-weighted lining fabrics that exhibited 45%, 40%, 69%, and 34% weight losses, respectively. The relatively new tin-weighted satin from Patricia Nixon's gown (1969) revealed only a 22% weight loss. The weighting of silk often results in “shattered” silk as evident by the lining fabric of Edith Roosevelt's gown (see fig. 3) showing the fiber fracture morphology associated with brittle materials (Bresee and Goodyear 1986; Hearle et al. 1989).
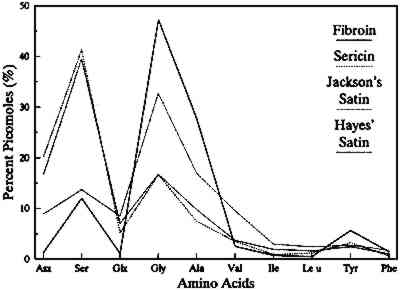
Compared to artificially aged silks, most of the First Ladies gowns have solubilities under denaturing conditions exceeding that of the 1000 kJ/m2 exposure to sunlight through glass (18%). Based on the artificially aged fabrics, 6.2 COMPOSITION OF THE EXTRACTED DEGRADATION PRODUCTSSince all the First Ladies silk gowns examined were soluble to some extent in 7M urea treatment, the composition of the proteinaceous material solubilized was analyzed. The amino acid compositions of the extracts from two of the gowns, Lucy Hayes's and Sarah Jackson's satins, revealed different protein constituents in the soluble material. As determined by postcolumn OPA IE-HPLC, the overall pattern of amino acids recovered from the extract of Sarah Jackson's satin is indicative of fibroin (i.e., large quantities of glycine and alanine with some serine) (fig. 4). It should be noted that the satin in Jackson's gown is probably early 20th century and consists of a silk warp–cotton weft. In contrast, the soluble extract from Lucy Hayes's satin (1877), which had a 45% weight loss, had an amino acid composition nearly identical to sericin (fig. 4).
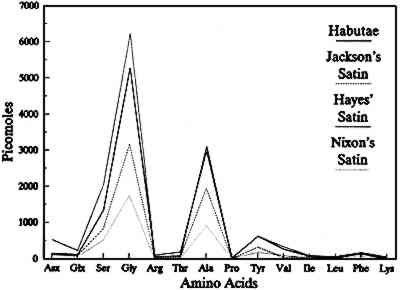
The presence of sericin in the fabric increases the loss of silk fabrics under denaturing conditions due to the solubility of this protein. The present data suggest that any denaturing solvent treatment, in addition to removing surface contamination, presents the possibility of removing some proteinaceous material, either sericin or fibroin, from the silk garment. The artificially aged fabrics show an incremental loss of the protein with solubilization in urea, first associated with the amorphous regions of fibroin and secondarily from both the amorphous and crystalline regions of the fibroin as the dose and ultraviolet content of the incident radiation increase (Becker and Tuross 1994). The percentage of serine in both the urea-extracted silk degradation products and the overall exposed habutae remained constant, at approximately 11%, indicating an even distribution of serine throughout both the amorphous and crystalline regions. Thus for our artificially aged habutae, the amorphous regions of fibroin are degraded first under mild exposure conditions and then, as the UV content and dose increase, the whole fibroin matrix—crystalline and amorphous regions—are affected. This result provides evidence for threshold effects in the deterioration process of silk fibroin and suggests a selective deterioration mechanism that becomes random as exposure to light persists. Selective destruction of amino acids has been reported for both thermal decomposition reactions (Vallentyne 1964) and photodegradative experiments (Meybeck et al. 1971; Becker and Tuross 1994). 6.3 OVERALL FABRIC AMINO ACID COMPOSITIONThe amino acid analyses of 41 different fabric samples from the First Ladies collection yielded amounts of glycine, alanine, and serine in the characteristic 4:3:1 ratio of Bombyx mori fibroin. The fabrics were therefore confirmed as consisting primarily of silk. Minor variations in the compositions could be due to the effects of impurities such as starch coatings, dyes, mordants, and weighting agents, which could result in artifactual losses or alterations during the hydrolysis prior to analysis (Hunt 1985). As with the solubility data, there is no temporal correlation between the quantity of amino acids recovered and attribution date, even for different fabrics from the same garment. In artificially aged silk, there is a decrease in the total number of picomoles detected by A comparison of the amino acid profiles of three First Ladies fabrics shows that these naturally aged silks fall into two populations, sericin-rich and sericin-depleted. The amino acid profile of Hayes's satin is high in serine and aspartic acid, and the tyrosine content is comparable to new habutae. Both serine and aspartic acid are present in larger quantities in sericin than in fibroin (fig. 5). The combination of the overall amino acid data with the composition of the material extracted indicates that Hayes's satin can be considered sericin-rich.
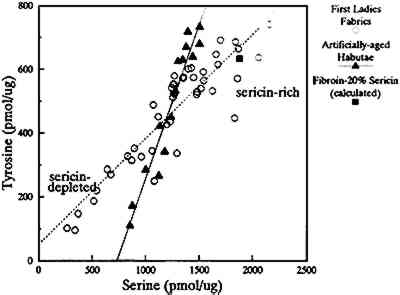
Sarah Yorke Jackson's and Patricia Nixon's satins, which exhibit low amino acid recoveries, are notably lower in serine, glycine, alanine, and tyrosine content than new habutae. However, some of the fabrics can contain other fibers. For example, Jackson's satin includes a cotton weft, which, affecting the initial starting weight of the fabric, can slightly decrease the overall recovery. But the analysis of the proteinaceous material within the fabric can be useful as an investigative tool to evaluate the presence or absence of sericin. The material extracted from Jackson's satin indicates an absence of sericin. The decrease in the amount of serine present in the overall amino acid composition of the First Ladies fabrics compared to modern silk habutae is evidence that the sericin has been removed and the fabric is at risk. Nixon's satin is a relatively modern fabric (1969) that has probably been subjected to extensive processing, including essentially complete removal of the sericin, similar to the modern habutae. Tyrosine is known to be effectively degraded by light (McLaren and Shugar 1964), and its loss from fibroin is evidence that photodegradation has occurred (Becker and Tuross 1994). The two amino acids, serine and tyrosine, serve as proxy for the presence of sericin (serine) and photooxidative damage (tyrosine), and these can serve as an analytical tool to assess the general biochemical state of the silk fabrics (fig. 6).
The artificially aged fabrics (pure fibroin) show a linear decrease in both amino acids with a slope of nearly one (r2 = 0.923, slope = 0.969). The tyrosine-serine (pmol/%g) data for the First Ladies fabrics show that some samples contain more serine and tyrosine, while others have less of both compared to the artificially aged silks. Again the high serine content is indicative of the presence of sericin. When evaluated by linear regression analysis, the tyrosine-serine ratios of the First Ladies fabrics are best described by a line (r2 = 0.822) with a much lower slope than the artificially aged fabrics. The First Ladies fabrics indicate a smaller loss of tyrosine per loss of serine, particularly for those fabrics with serine contents greater than those of the habutae. Calculated tyrosine-serine points for compositions of fibroin with 20% and 30% sericin fall in the upper-right-hand corner of figure 6. Those amino acid compositions that show a serine content higher than the pure fibroin habutae have sericin associated with the fabric. Furthermore, tyrosine appears to be well preserved when a large serine content is present, probably due to the protective coating effect of sericin. Photodegradation is initially a surface phenomenon; thus the sericin coating could provide a screen for incident light or increase the length of the induction period before destruction of the main peptide chain by cleavage. 7 SUMMARY AND CONSERVATION IMPLICATIONSBiochemical characterization of a proteinaceous textile, particularly silk, not only identifies the major constituents of the fabric but also indicates the state of preservation of the material. All the historic fabrics exhibited some degree of degradation, as evidenced by their solubility in denaturing conditions and the composition of the solubilized degradation products. An overall decrease in all the amino acids recovered, particularly tyrosine, can be used to relate a historic silk to artificially aged fibroin. Once an artificially aged sample, comparable to the actual object, has been prepared, it can then be used in lieu of the object in the evalution of prospective treatments. The compositional data from the silk fabrics of the First Ladies Collection revealed two populations; sericin-rich and sericin-depleted materials. These two populations present different concerns to the conservator. While sericin yellows easily, it protects the fibroin filaments against light damage as exhibited by the state of protein preservation of the sericin-rich fabrics. The naturally aged fabrics that retain a sericin coating are at great risk of losing that protective coating with any wet treatment. Conversely, the sericin-depleted fabrics are more likely to have already suffered severe light damage, and their increased solubility is a result of actual fibroin loss. Any conservation treatment that involves washing sericin-depleted fabrics not only jeopardizes the integrity of the object but provides an opportunity for further light damage to the newly exposed fibroin. The analytical techniques described require an extremely small amount of fabric that can easily be sampled from the unfinished edge of an inside seam. Spot treatments of fabrics to be cleaned with any solvent can be examined for material removed in the washing solution. Amino acid analysis of the cleaning solution can determine not only how much material is lost but also the nature of the protein being removed. The aggregate data from the analysis of the First Ladies costumes indicate that silk fabrics manufactured in this century are likely to have been extensively processed, resulting in a complete loss of the sericin coating. This processing puts these recently produced fabrics at extreme risk for light damage. ACKNOWLEDGEMENTSThis study was funded by a Smithsonian Pre-Doctoral Conservation Science Fellowship (M.A.B.) in conjunction with the Johns Hopkins University. REFERENCESAtlas Electric Devices. 1986. Atlas Ci-65 Weather-ometer instruction manual. Chicago, Ill.: Atlas Electric Devices. I–17. Becker, M. A.1993. The characterization of the initial degradative alterations of Bombyx mori silk fibroin. Ph.D. diss., Johns Hopkins University, Baltimore, Md. Becker, M., and N.Tuross. 1992. Novel approaches for assessing the preservation of historic silks: A case study of the First Ladies' gowns. Materials Research Society Bulletin17(1): 39–44. Becker, M. A., and N.Tuross. 1994. Initial degradative changes found in Bombyx mori silk fibroin. In Silk polymers, ed.D. L.Kaplan, W. W.Adams, B.Farmer, and C.Viney. Advances in Chemistry series 544. Washington, D.C.: American Chemical Society. 252–69. BensonJ. R., and P. E.Hare. 1975. O-Phthalaldehyde: Fluorogenic detection of primary amines in the picomole range—Comparison with fluorescamine and ninhydrin. Proceedings of the National Academy of Science USA72(2): 619–22. Bidlingmeyer, B. A., S. A.Cohen, and T. L.Tarvin. 1984. Rapid analysis of amino acids using pre-column derivatization. Journal of Chromatography336: 93–104. Bogle, M. M.1979. The deterioration of silks through artificial weighting. In Textile Conservation Center Notes, no. 11. North Andover, Mass.: Merrimack Valley Textile Museum. Bresee, R. R., and G. E.Goodyear. 1986. Fractography of historic silk fibers. In Historic textile and paper materials, ed.H. L.Needles and S. H.Zeronian. Advances in Chemistry series 212. Washington, D.C.: American Chemical Society. 95–109. Cohen, S. A., B. A.Bidlingmeyer, and T. L.Tarvin. 1986. PITC derivatives in amino acid analysis. Nature320: 769–70. Cooper, T. G.1977. The tools of biochemistry. New York: John Wiley and Sons. Hare, P. E.1977. Subnanomole-range amino acid analysis. In Methods in enzymology, vol. 47, part E, ed.C.H.M.Hirs and S. N.Timasheff. New York: Academic Press. 3–18. Hearle, J.W.S., B.Lomas, W. D.Cooke, and I. J.Duerdon. 1989. Fibre failure and wear of materials. New York: John Wiley and Sons. Howitt, F. O.1946. Bibliography of the technical literature on silk. New York: Hutchinson's Scientific and Technical Publications. Hunt, S.1985. Degradation of amino acids accompanying in vitro protein hydrolysis. In Chemistry and biochemistry of the amino acids, ed.G. C.Barrett. New York: Chapman and Hall. Chapter 12. Komatsu, K.1980. Chemical and structural characteristics of silk sericin. In Zoku Kenshi no Kozo, ed.N.Hojo. Ueda, Japan: Shinshu University. 379–415. Lucas, F., J.T.B.Shaw, and S. G.Smith. 1958. The silk fibroins. In Advances in protein chemistry, ed.C. B.AnfinsenJr. et al. New York: Academic Press. 108–242. Lucas, F., J.T.B.Shaw, and S. G.Smith. 1960. Comparative studies of fibroins. Part 1, The amino acid composition of various fibroins and its significance in relation to their crystal structure and taxonomy. Journal of Molecular Biology2: 339–49. McLaren, A. D., and D.Shugar. 1964. Photochemistry of proteins and nucleic acids. New York: Pergamon Press. Meybeck, A., B.Gurtner, H.Balard, R.Morvan, J. L.Huron, and J.Meybeck. 1971. Photochemistry of peptides in solution. In Proceedings of the fourth international Wool Textile Research Conference, Part 1, ed.L.Rebenfeld. Applied Polymer Symposium 18. New York: John Wiley and Sons. 325–31.
Robinson, A. B., and Rudd, C. J.1974. Deamidation of glutaminyl and asparaginyl residues in peptides and proteins. Current Topics in Cell Regulation8: 247–94. Rudall, K. M.1960. Silk and other cocoon proteins. In Comparative biochemistry, 2d ed., vol. 4b, ed.M.Florkin and H. S.Mason. 397–433. Smithsonian Institution. 1914. Annual report of the National Museum. Washington D.C.: Smithsonian Institution. 23–24. Smithsonian Institution. 1919. Annual report of the National Museum. Washington D.C.: Smithsonian Institution. Smithsonian Institution. 1931. Annual report of the National Museum. Washington D.C.: Smithsonian Institution. Stathoplos, L.1989. Amino acids in planktonic foraminiferal tests. Ph.D. diss. University of Rhode Island, Kingston, R.I. Stryer, L.1988. Biochemistry. New York: W. H. Freeman and Company. 220–21. Vallentyne, J. R.1964. Biogeochemistry of organic matter. Part 2, Thermal reaction kinetics and transformation products of amino acids. Geochimica et Cosmochimica Acta28: 157–88. FURTHER READINGCarboni, P.1952. Silk: Biology, chemistry and technology. London: Chapman and Hall. Hare, P. E.1964. Amino acids in organic geochemistry. Carnegie Institute of Washington64: 232–35. AUTHOR INFORMATIONMARY A. BECKER is a Science and Technology Agency / National Science Foundation fellow at the National Institute of Sericultural and Entomological Science in Tsukuba, Japan. She received the first Ph.D. specializing in conservation science in the Materials Science and Engineering Department at the Johns Hopkins University. She received a B.S. in chemical engineering from the University of Rochester and an M.S. in textile engineering from North Carolina State University. Address: Department of Silk and Biopolymer Technology, National Institute of Sericultural and Entomological Science, 1-2 Ohwashi, Tsukuba-shi, Ibaraki-ken 305, Japan. POLLY WILLMAN is the senior textile conservator in the Department of Conservation at the Smithsonian Institution's National Museum of American History. She holds a B.S. in textile science and an M.A. in historic costume and preservation, both from Colorado State University. She was responsible for coordinating the conservation of the costumes in the First Ladies Collection. Address: BB017, National Museum of American History, Smithsonian Institution, Washington D.C. 20560. NOREEN C. TUROSS is a research biochemist at the Smithsonian Institution's Conservation Analytical Laboratory. After receiving her Ph.D. from Brown University in 1985, she was a postdoctoral fellow at the Bone Research Branch of the National Institutes of Health. She is interested in the degradation of biomolecules in the museum environment and in the fossil record. Address: Conservation Analytical Laboratory, Smithsonian Institution, Washington D.C. 20560.
 Section Index Section Index |