CLEANING, IRON STAIN REMOVAL, AND SURFACE REPAIR OF ARCHITECTURAL MARBLE AND CRYSTALLINE LIMESTONE: THE METROPOLITAN CLUBFRANK G. MATERO, & ALBERTO A. TAGLE
4 CONSERVATION PROGRAM4.1 EXAMINATION AND ANALYSIS In 1985, the Center for Preservation Research, Columbia University (Frank G. Matero, director) undertook preliminary examination of the exterior for James Stewart Polshek Associates. In 1989, the club began full study of the exterior stone masonry in preparation for exterior cleaning. In addition to the archival
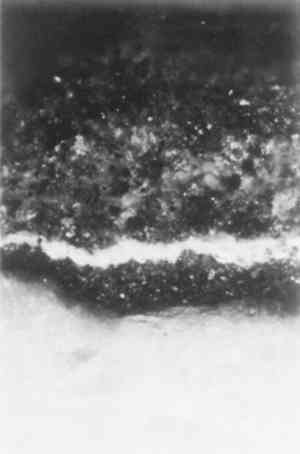
Samples of both marble and limestone taken from protected (i.e., rain-sheltered) areas also revealed thick black gypsum crusts with embedded airborne particulates of carbon soot, fly ash, iron, and other metals typically found on calcareous stones in polluted urban environments (figs. 6–8). Such crusts were most prevalent on the south elevation, where the narrow street, surrounding tall buildings, and heavy traffic all contributed to the formation and retention of pollution-related soiling and sulfate crusts under and over the paint layers.
Weathering of each stone type was assessed using thin-section petrography and scanning electron microscopy as well as by visual inspection on site. At high magnification all exposed samples exhibited surface etching and cracking of crystal faces, conditions that can be attributed
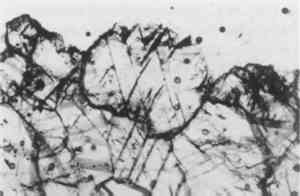
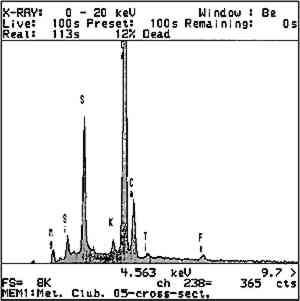
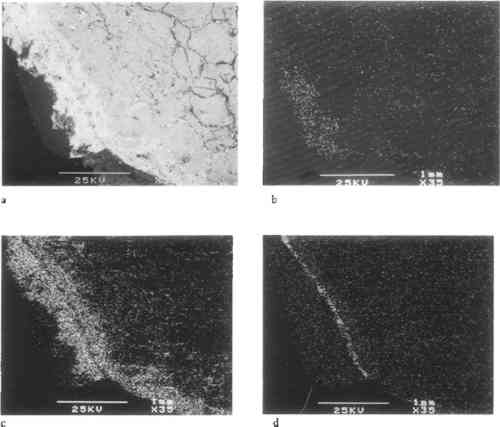
Deterioration of the dolomitic crystalline limestone was restricted to the carved details and arises of protected elements subject to water saturation and slow drying, such as the balcony consoles and soffit moldings. In these locations, disaggregation of the stone was observed in cross section as microcracking along the grain boundaries in association with visible crystalline deposits. The localized concentration of sulfur detected by SEM-EDX in a layered structure on the surface and along the detached grain boundaries suggests the presence of gypsum formed by the action of atmospheric SO2, both as a complex crust and within the grain structure as cryptofluorescence, the latter a result of capillary migration in a humid environment (fig. 11). The internal formation of sulfates within the stone's upper matrix due to oxydation of pyrites increases this microcracking and resultant disaggregation; the condition undoubtedly has been worsened by freeze-thaw cycling as a result of increased water permeability and surface area and decreased intergranular cohesive strength.
In addition to these deterioration phenomena, the crystalline dolomitic limestone exhibited unique widespread staining, ranging in color from pale orange to deep rust red. Staining appeared to be greatest in those areas of heavy water exposure and soaking, such as the water table and windowsills. In thin-section examination under normal and polarized transmitted light, this discoloration appears as a fairly even zone extending down 2 mm from the surface with distinct darkened grain boundaries (see fig. 4). This dark intergranular material can be identified using SEM-EDX as iron, presumably originating from the naturally occurring pyrite (iron sulfides) and hematite (iron-III oxide), commonly reported as intrinsic and altered accessory minerals in this stone (see fig. 11). The general mechanism for the type of intrinsic ferruginous staining observed in the crystalline limestones of the Metropolitan Club has been described in the literature as the diffusional transport of colloidal particles formed by the oxidation of ferruginous mineral inclusions In an outdoor environment, the limestone is subject to two main agents: acidic water and oxygen, which will affect the iron-bearing accessory minerals in the stone in several ways, simplified as:
All together, the humid atmosphere and the acidic environment, regardless of the effect caused to the stone matrix, will create the conditions for the iron stains, which can be expressed in the final equation:
In this case, hydrated ferric hydroxides and ferric sulphates are responsible for the iron-based discolorations. This is a simplified explanation of a very complex natural phenomenon that does not take into account the stability of the formed compounds in the medium (stone) or any other possible interactions. Nevertheless, it provides a reasonable explanation for the intrinsic iron staining observed on the crystalline limestones. In addition, the resulting acid in the calcite matrix will react to form gypsum:
In its own way, hematite will hydrate as:
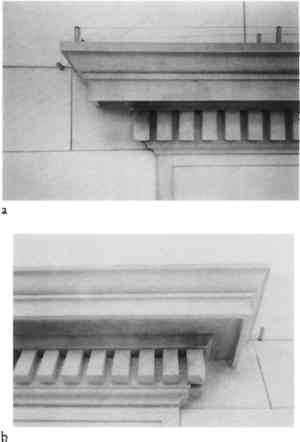
Metallic staining, especially on light-colored stone, is generally considered to be one of the most difficult cleaning problems encountered for porous building materials. Such stains, regardless of their metallic species, can be attributed to either extrinsic or intrinsic sources. Extrinsic metallic staining is most often due to the corrosion of associated architectural metalwork, especially of iron, copper, and the copper alloys brass and bronze. These elements generally include internal anchors, pins, rebar, or associated features such as roofs, flashing, or decorative grilles. Also included is staining resulting from the deposition of metallic particulates found in most airborne pollution and constituting an average of approximately 10% (by weight) of urban grime or dry deposition (Cutter and Kissa 1987). Such staining was observed at the Metropolitan Club on protected ashlar surfaces of the Vermont marble in combination with thin surface crusts of gypsum and carbon soot. Intrinsic sources of metallic staining are usually the accessory or secondary minerals of the stone itself, which can occur as isolated inclusions or as distributed material in the crystalline matrix, as in the case of the Metropolitan Club's crystalline limestones. Obviously intrinsic sources of metallic staining present far greater problems in their treatment and control given the difficulty of removing or controlling the source. 4.2 PAINT REMOVAL AND CLEANINGBefore an evaluation of paint removal techniques could be made and the existing condition of the stone established, in situ tests with a variety of commerical alkaline and organic solvent paint strippers were first performed. These tests were executed in various locations to observe product performance and to determine the most appropriate dwell times, application conditions, and residue removal and collection procedures for the different stones. The contractor selected a potassium hydroxide-based commercial paint stripper (Deidrich 606X Paint Stripper) as the most expedient means of completely removing all layers of overpaint. One application of 3–4 hours was followed by a pressure water rinse of 400–600 psi. After removal of the paint stripper residue, any remaining potassium hydroxide was neutralized with a rinse of dilute phosphoric and acetic acids (Sure Klean Limestone Afterwash). After full removal of the paint, localized soiling and staining of varying degrees (suggested by the sample cross sections and early photographs) was revealed, helping to explain the club's initial decision to paint the building in 1967 (fig. 12). Although the records suggest that the building was steam cleaned prior to painting, the irregular black soiled surface under the paint clearly indicated that the process was either bypassed or poorly executed. Beginning with the most basic of techniques, all stone surfaces were washed with a low-volume, low-pressure (1–2 gals/min.) water spray for generally 24 hours with city water filtered through an industrial in-line particulate water filter as a precaution against iron particulate contamination from aged water pipes. Contact time was occasionally longer depending on the severity of the soiling and the thickness of the protected crusts. Because of the potential danger of water damage to the club's elaborate interiors through exterior leaks, all windows were temporarily covered with polyethylene-covered frames, and all open joints were temporarily sealed prior to paint removal and aqueous cleaning. Partial repointing was undertaken only after cleaning to ensure an accurate match with the existing mortar.
The water spray cleaning performed effectively in removing the majority of the overall black soiling (occurring on 30–40% of the total building surface), especially from the Vermont marble (see fig. 12). Complete removal of the soiling and much of the ligthter ferruginous 4.3 IRON STAIN REMOVALThe removal of obscuring paints and disfiguring soiling and crusts revealed what cross sections had already suggested: large areas of orange ferruginous staining on much of the crystalline limestone. Metallic stains and their removal from masonry represent a problem chemically different from that of the surface soiling and encrustation described above. One of the best approaches for the removal of such deep-seated stains is the use of chemical complexing or sequestering agents. These compounds will react with the metallic ion responsible for the discoloration to yield a stable, soluble species that can diffuse into the solvent and be removed when the liquid phase is soaked up or flushed away. The ideal agent should react rapidly with the metallic stain to yield a stable complex compound and not form a soluble complex ion or a soluble substance with any of the constituents of the stone, such as calcium or magnesium. In addition, any such complexing agent under consideration should be of relatively low cost for large-scale building application. Complexing agents are not new to conservation practice. Such compounds have been used for selective cleaning, desalination, stain removal, and treatment reversal for many years on small museum objects (Richey 1975; Chartier 1991). Chelating agents such as EDTA (ethylenediaminetetraacetic acid) have been used for rust reduction, salt removal, and iron and copper stain removal from stone, leather, bone, and textiles and for the removal of accretions from archaeological ceramics and glass. Use of ammonium citrate for conservation treatments has been tested and reported to clean rust from metallic artifacts, especially when the presence of chlorides must be avoided (Mibach and Organ 1972). For the removal of iron stains from stone, sodium citrate has been reported in use as early as 1957 (Amoroso and Fassina 1983), EDTA (Detarol) in 1962 (Plenderleith 1971), and citric acid, oxalic acid, and EDTA in 1976 (Rinne 1976). Stambolov (1968) addressed the problem specifically for calcareous stone, citing only sodium citrate among other compounds as effective. Perhaps the most insightful early discussion of the mechanisms of ferruginous staining on stone was published by Lewin and Rock (1976). Through experimental models, they assessed the effects of phosphates, fluorides, oxalates, and salicylates as complexing agents for hydrous iron-oxide staining on stone. According to their results, none of these compounds proved desirable for carbonate stones due to their poor efficiency or attack on the stone. Experiments on the treatment of discolored concrete (Greening and Landgren 1966), as well as rust removal from weathered Indiana limestone (Gale and Weiss 1982) using ammonium citrate, cited good results. However, the process can be dangerous to carbonate rocks, as ammonium citrate has an acidic hydrolysis. The effectiveness of the ammonium citrate salt in comparison with other citrate salts, especially when buffered with ammonium hydroxide for use on acid-sensitive carbonate rocks, has never been adequately addressed or explained. In fact, when applied in an alkaline solution (pH 9.0) as a poultice prepared with ammonium hydroxide or following potassium hydroxide cleaning treatments, improved stain removal has The formation of ferric-ammonium citrate can be outlined as follows. Iron forms two different types of chelates with citric acid. Iron-II ions form very unstable complexes, which oxidize easily into iron-III compounds. These compounds can be of two types, one of reddish brown color in a basic medium, [Fe3(C6H5O7)2(OH)2(H2O)2]3C6H5O7.18H2O; and one of greenish color in an acidic environment, H6[Fe2(C6H4O7)3]. Sodium citrate in the poultice medium forms two types of complexes, one of greenish color in an acidic medium, Na3H3[Fe2(C6H4O7)3], and one of reddish brown color upon addition of sodium hydroxide, turning the medium basic, Na3Fe2(C6H5O7)2. The presence of ammonia allows the formation of different compounds. Iron-II builds unstable complexes that oxidize easily into the iron-III compounds. These compounds can be of two types, which are highly soluble in water in contrast to the ammonium-free compounds. Depending on the basicity of the medium (the hydrolysis of ammonium citrate is slightly acidic), the following green complex is formed: {Fe[C6H5O7(FeOH)][C6H4(FeO)O7(NH4)2](H2O)2}3C6H5O7.6H2O. Increasing the amount of ammonia in the solution forms a reddish brown complex, {Fe[C6H4(FeO)O7(NH4)2]2(H2O)2}3C6H5O7.�H2O, when the medium is clearly ammoniacal. The presence of both green and reddish brown complexes was confirmed in an experiment designed to assess the comparative efficacy of ammonium and sodium citrate at varying pH with and without ammonium hydroxide (fig. 13). Six samples of a naturally weathered, medium-grained, nearly pure calcitic marble were artificially stained by soaking them in a solution of hydrated iron-II sulfate (copperas), which quickly oxidized to a deep orange-brown iron-III sulfide. The following stain removal tests were then performed with poultices of Fuller's Earth mixed with saturated solutions of ammonium and sodium citrate prepared with deionized water at room temperature (24�C).
Sample 1: Control (no treatment) Sample 2:Ammonium citrate with sodium hydroxide (pH 9.0) Sample 3:Ammonium citrate with ammonium hydroxide (pH 9.0) Sample 4:Ammonium citrate with ammonium hydroxide (pH 7.0) Sample 5:Sodium citrate (pH 9.0) Sample 6:Ammonium citrate with ammonium hydroxide (pH 8.0) Each poultice was applied three times to each sample; each application was covered with plastic film to ensure a consistent dwell time of 24 hours and then unwrapped and allowed to air dry before mechanical removal and rinsing of the poultice. The results of the laboratory tests confirm what had been observed in the field. Samples 2 and 3, both treated with ammonium citrate at a pH of 9.0, generally displayed the best stain removal. Of these, sample 3 showed the greatest improvement in stain reduction. Sample 5, prepared with sodium citrate at a pH of 9.0, affected some stain reduction but induced flaking damage to the stone as a result of the recrystallization of sodium salts. Ammonium citrate in an alkaline ammonia solution appears to be the safest and most effective method of applying citrates to eliminate iron stains in carbonate rocks. The better performance of the ammoniacal ammonium citrate medium can be explained considering the following points:
As a cleaning method for ferruginous material in carbonate rocks, ammoniacal ammonium citrate poultices also offer a safer pH range (pH 9.0) so as not to allow the acidity of the remaining citric acid to attack the carbonates of the stone. Sodium citrate, the more 4.4 FIELD APPLICATIONThe translation of custom formulations created in the laboratory into wide-scale application can be a difficult process, especially if the processes involved in their manufacture and use are sensitive, labor intensive, or hazardous. Fortunately, the use of an ammoniacal citrate poultice was feasible for this critical phase of cleaning due to its efficacy and ease in preparation, application, and relatively low cost. This availability was important, since no other commercial cleaning products formulated for the treatment of metallic, specifically iron, stains were found to be as safe or effective as the ammonium citrate-ammonium hydroxide treatment. Successful treatment of the stains, however, did require the preparation of detailed specifications for the controlled preparation and application of the poultices (see appendix A) and the creation of mock-ups, or controls, on site to establish a norm and cleaning reference for the contractor (fig. 14).
Large-scale poultice cleaning proved to be a highly effective and controllable technique for removal of the most severe iron staining on the crystalline limestone elements. The poultice was prepared on site and trowel-applied as a paste 1/4 in. thick, then covered with polyethylene film to increase dwell time and reduce evaporation. After 24 hours, the film was removed and the poultice allowed to dry before mechanical removal with plastic or wooden scrapers. After removal and containment of the dried poultice, all surfaces were rinsed with a water spray. In most locations, one application was sufficient to remove the staining. Only in heavily stained locations, such as the water table and window lintels and sills, were multiple applications required to reduce the staining to an acceptable level (fig. 15). Where areas of staining were large and flat, the poultice could be quickly applied as a slurry with a pressurized sprayer normally used for applying Gunite.
This method of stain removal could be easily controlled to produce a controlled level of cleaning that avoided the often common problems of selective overcleaning and the creation of an irregular variegated surface. In addition, 4.5 CONSOLIDATION AND MECHANICAL REPAIRDespite the severity of the soiling and staining, the majority of the exterior marble and limestone was in sound condition, with only minor superficial sugaring of the Vermont marble surfaces and localized friability of certain crystalline limestone details (see above). Only the 23 attic story garlands of Vermont marble were found to be deteriorated enough to require consolidation treatment with an ethyl silicate consolidant (ProSoCo Conservare OH Stone Strengthener) after cleaning and prior to the filling of cracks and losses. Each element was first carefully dry-brushed to remove loose surface debris, and the consolidant was brush-applied in two cycles of three applications per cycle. A final application of methyl ethyl ketone was made to prevent surface gloss, following the supplier's instructions. The treated areas were protected from rain by a polyethylene sheet for two weeks during cure. After cure, all cracks and small losses were filled to prevent water access and restore some structural support to eroded areas with a mortar of white Portland cement, lime putty, and fine white banding sand. Although the majority of the carved surfaces of the crystalline limestone were quite sound and the tooling still crisp, small incipient cracks and spalls were detected after inspection at close range by tapping with a small acrylic mallet. These flaws–often observed in association with the phlogopite inclusions and possibly exacerbated by carving stresses–caused the entrapment of water, which in turn has induced further damage by freeze-thaw cycling and the growth of microflora. This condition has resulted in the detachment and occasional loss of carved surface details. In addition to the obvious aesthetic disfiguration, this deterioration posed a potential safety hazard, especially in the upper stories overlooking the street. As a result, every carved element was hand-surveyed and all cracked surface details were carefully removed, cleaned, and degreased with water, alcohol, and finally acetone and reattached with a two-part polyamide epoxide adhesive (Sika HiMod Gel 2 part epoxy). Only in a few cases, where the fragments were large or the cracks occurred in structural elements, such as the entrance columns, were threaded stainless steel pins required for resetting in combination with the epoxy adhesive. These repairs as well as all surface cracks were filled with the same mortar mix described above for the garlands. When larger fills were required to visually reintegrate areas where previous cement patches of poor quality were removed, several mortar mixtures of white Portland cement, lime putty, and a blend of yellow and white sands with some pigment were employed to match both the color and texture of the cleaned crystalline limestone. Blended sands provided good color, texture, and replication of the specular reflection characteristic of the large grained crystalline limestone. During the project, it was discovered that several of the balusters and top rail components of the balconies had been replaced with poor concrete substitutes at some time in the past. Due to the unavailability of the crystalline limestone or any other suitable stone match, cementitious cast stone replacements were fabricated from molds utilizing the same blended yellow and white sands and pigments used for the fills. The replacements are an excellent match to the cleaned, weathered originals. Although water was identified as a major contributing factor in the formation of iron staining, water repellents were not applied to the stone after cleaning. This decision was made due to the encroaching cold weather and the limitations that hydrophobic treatments
|