SHORT COMMUNICATION A NOTE ON THE IMPRIMATURA IN TWO OF DOSSO DOSSI'S PAINTINGSBARBARA H. BERRIE
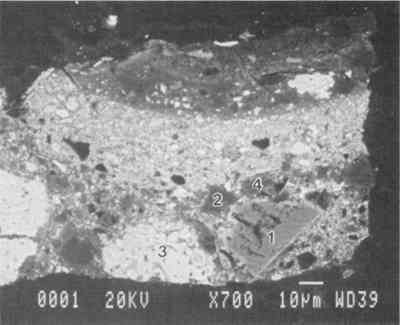
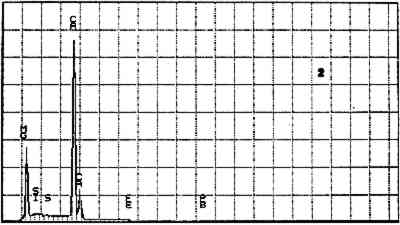
ABSTRACT—The imprimatura in two paintings by Dosso Dossi (Ferrarese, ca. 1490–1542) were examined using scanning electron microscopy coupled with energy dispersive x-ray spectrometry. An assemblage of mineral species was found and identified, among them pyrite, dolomite, micaceous minerals, gypsum, and quartz. A high proportion of lead white was also found. The results of the analysis suggest that clay was used in the imprimatura mixture. A search of the literature on painting technique of the time yielded little evidence about the use of clay in the imprimatura layer, although it is discussed in Vasari in the 16th century. During a study of the materials and methods used by the artist Dosso Dossi (ca. 1490–1542), an unusual m�lange of materials in a second ground layer, or imprimatura, was discovered in some of the pictures. This layer was studied in cross sections from two of the paintings. The findings described in this note will be addressed in more detail in a forthcoming article that will also present the other materials and methods used by Dosso. Dosso Dossi painted at the Este court in Ferrara, Italy, from 1514 until his death in 1542. The court was rich and a proud patron of the arts. Alfonso I d'Este, the third duke of Ferrara (1505–34), commissioned many works of art from the leading artists of the time, including Titian, who painted at the court several times between 1519 and 1530. Although Dosso was stylistically influenced by his Venetian contemporaries, their influence on his painting method is not yet known. Five paintings by Dosso have been studied to begin to characterize his materials and methods and to compare them to traditional Ferrarese and Venetian practice (Berrie and Fisher 1993). Cross sections obtained from the paintings were mounted in a polyester resin and polished on silicon carbide grit papers and cloths. The sections were examined using optical microscopy and then coated with carbon and analyzed using scanning electron microscopy–energy dispersive spectroscopy (SEM-EDS). The data were obtained using a Tracor Northern (now Noran) detector on a JEOL 840A SEM or an Oxford Super ATW detector on a JEOL 6300 SEM. In all five paintings examined, whether on wood or textile, the preparatory ground layer applied directly on the support was gesso prepared from burnt gypsum bound in a proteinaceous glue. Staining the sections with neutral amido black showed that the gesso was sometimes sealed with a layer of glue or size. The practice of sealing the gesso with size before applying the imprimatura was described by Giorgio Vasari ([1568] 1966) and has been noted in many contemporary Italian paintings. Surface examination revealed that there is a cool gray-brown or dark gray layer above the gesso ground in all the paintings examined. This layer appears to cover the entire area of the support. The particle mixture is inhomogeneous and varies somewhat in appearance from sample to sample. The back-scattered electron image (BSE) of a cross
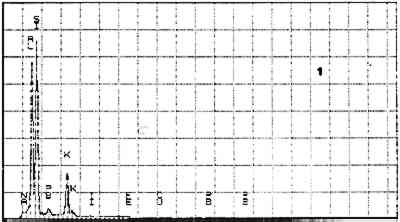
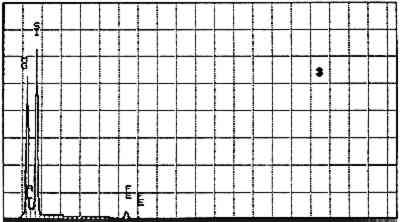
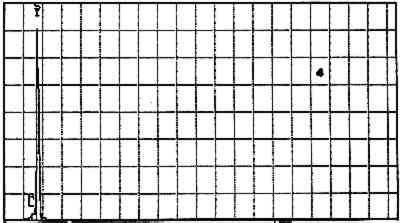
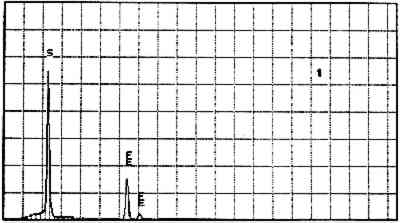
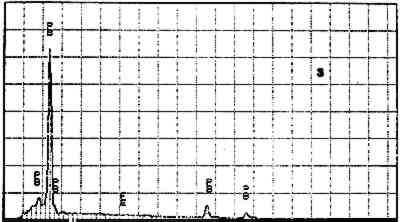
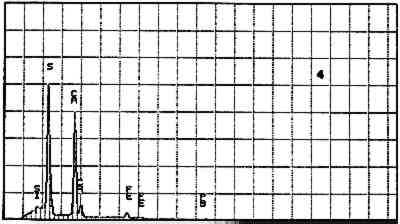
Elements Present from SEM/EDS Analysis and Possible Mineral Identity of Particles in the Imprimatura in the Cross Section from Circe and Her Lovers in a Landscape A section from a soldier's orange jacket in The Battle of Orlando and Rodomonte (Wads-worth Atheneum, Hartford, Conn., mid-1520s) was similarly analyzed. The BSE image of it is shown in figure 6. EDS spectra of the noted particles are given in figures 7–11. The elements present in the particles and their possible mineralogical identities are given in table 2. The identification of specific minerals should be considered tentative since several mineral species could have similar chemical composition.
Elements Present froim SEM/EDS Analysis and Possible Mineral Identity of Particles in the Imprimatura in the Cross Section from The Battle of Orlando and Rodomonte The white lumps seen in the cross sections are aggregates of the pigment lead white. The other materials identified in the imprimatura are not generally considered pigments: pyrite (FeS2) is found in many kinds of rocks. It is frequently The use of colored grounds or imprimaturas has been noted, especially in paintings dating from the mid-16th century. These grounds are most often red or yellow, though gray grounds have been found in some paintings (Lazzarini 1987). The gray ground found in Dosso's work plays the same role as other colored imprimaturas: it provides an overall color to the painting support. However, it appears that Dosso used a siliceous earth rather than an iron earth for this imprimatura. These earths were readily available materials, easily and inexpensively prepared for use by levigation. There is little evidence, either from the literature or from technical analysis, of the use of siliceous earths in imprimaturas. Since their preparation for various uses is so simple, it may not have warranted much attention by authors of treatises on painting technique. Nonetheless, Vasari's ([1568] 1966, 230–31) description of the preparation of imprimaturas does allude to the use of clay:
The specific material that Vasari mentions is unknown, although other writers also addressed the special requirements of bell casting. Theophilus ([12th c.] 1979) describes using layers of thin shells of clay for casting bells. The practice appears little changed until the 16th century, when Biringuccio ([1540] 1966) describes a similar process of bell casting. In 1568 Benvenuto Cellini ([1568] 1967, 113) described the clay used for fabricating the cast:
It might be noted that Cellini's description reveals that a wide diversity of clays was suitable for bell casting. Clays are composed of extremely fine particles of aluminosilicate minerals. They often also contain so-called nonclay minerals such as quartz, calcite, feldspar, and pyrite. Thus, the results show that one of the clay types Cellini describes could be the material Dosso used in his imprimatura. This investigation is the first that has identified an impure clay as an ingredient in the imprimatura of any artist. Although the specific minerals in the samples from Circe and Orlando and Rodomonte are different, all are indicative of clay mixed into the preparation. The small sample size means the material cannot be fully characterized, and differences and similarities cannot yet be established. However, these results show that more careful analysis of the imprimatura reveals unexpected materials. The earth used by Dosso undoubtedly affects the appearance of his paintings. Analysis of his paintings will specifically examine the possibility of the use of clay imprimaturas as described by Vasari. ACKNOWLEDGEMENTSStephan Kornhauser, Wadsworth Atheneum, Hartford, Connecticut, generously provided samples from The Battle of Orlando and Rodomonte. The contributions of Melanie Feather and Camie Campbell of the Conservation Analytical Laboratory, Smithsonian Institution, are gratefully acknowledged. They provided much assistance with the SEM-EDS analysis, which could not have been accomplished without them The author also thanks William Melson, Mineralogical Science Department, National Museum of Natural History, Smithsonian Institution, and Sarah L. Fisher, National Gallery of Art, for very helpful discussions. REFERENCESBerrie, B. H., and S. L.Fisher. 1993. A technical investigation of the materials and methods of Dosso Dossi. ICOM Committee for Conservation Preprints, 10th Triennial Meeting, Washington. Paris: ICOM. 1:70–74. Biringuccio, V. [1540] 1966. The pirotechnica. Translated by C. S.Smith and M. T.Gnudi. Cambridge: Cambridge University Press. Cellini, B. [1568] 1967. The treatises of Benvenuto Cellini on goldsmithing and sculpture. Translated by C. R.Ashbee. New York: Dover. Deer, W. A., R. A.Howie, and J.Zussman.1966. An introduction to the rock-forming minerals. London: Longman. Lazzarini, L.1987. The use of color by Venetian painters 1480–1580: Materials and techniques. In Color and technique in Renaissance painting: Italy and the North, ed.M. B.Hall. New York: J. J. Augustin. 115–36. Theophilus. [12th c.] 1979. On divers arts. Translated by J. G.Hawthorne and C. S.Smith. New York: Dover. Vasari, G. [1568] 1966. On technique. Translated by L. S.Maclehose. New York: Dover. AUTHOR INFORMATIONBARBARA H. BERRIE received a B.Sc.(Hons) from St. Andrews University, Scotland, in 1977 and a Ph.D. in inorganic chemistry from Georgetown University in 1982. She held a National Research Council Research Associateship at the Naval Research Laboratory from 1982 to 1984. In 1984 she joined the Scientific Research Department of the National Gallery of Art, where she is senior conservation scientist. Address: National Gallery of Art, Washington, D.C. 20565.
 Section Index Section Index |