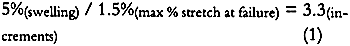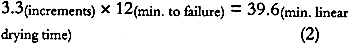PHYSICAL AND MECHANICAL PROPERTIES OF ALBUMEN PHOTOGRAPHSTIMOTHY VITALE, & PAUL MESSIER
4 DISCUSSION4.1 SOURCE OF ALBUMEN CRACKING4.1.1 Propagation of Albumen CracksAll albumen prints examined by the authors, including the experimental albumen print, were observed to be cracked. Thus, the mechanical properties used to predict the behavior of albumen prints are those from the bars with pre-existing cracks. Figure 3 shows that unsupported nonsensitized albumen with pre-existing cracks can be strained only 1.5% before failure occurs and that the tensile strength is a very low 62 psi. Figure 7 shows that unsupported sensitized albumen swells 12% when the humidity is increased from 50% RH to 85% RH, and 17%+ when immersed. Figure 5 shows the behavior of albumen on paper: the historical albumen prints swelled 1.7% (CD) when wet. When these materials shrink, the strain limit of the albumen will be exceeded, and cracking will result. It is important to note that the bars were nonsensitized albumen, while the prints are sensitized albumen. Substantial evidence exists that albumen is physically hardened and rendered somewhat insoluble by sensitization. While it is not possible to test directly, the effect of hardening on mechanical properties may not be significant because the adsorption-desorption behavior of the sensitized experimental albumen print is not markedly different from that of the nonsensitized albumen/paper composite. 4.1.2 Direction of CrackingAll albumen prints have cracks. Even modern commercial (Chicago Albumen Works) unsensitized albumen papers exhibit cracking (Messier 1991). It was observed in the treatment-based experiment (Messier and Vitale 1994) that 14 of 20 historical albumen prints had a predominant crack pattern that ran along the machine direction of the paper. Since cracks propagate 90� to applied stress, it can be concluded that the albumen layer experiences greater stress in the cross-machine direction of the paper base. A possible explanation for this observation can be found in the sorption-desorption data for unsupported sensitized albumen (fig. 7). During the desorption phase of the print (fig. 6a), the paper base is slightly more expanded in 4.2 RATE-OF-STRAIN–DEPENDENT PROPERTIES OF UNSUPPORTED NONSENSITIZED ALBUMENThe differences in moduli caused by differences in strain rate, combined with observations based on physical handling of the material, indicate that albumen has pronounced rate-of-strain–dependent properties. In the fast strain rate domain it is brittle, and in the slow domain it is rubbery. Under common museum conditions where humidity or water content change slowly, cracked albumen acts like rubber, offering low resistance to stress or strain and yielding a normal amount before failure. When sudden changes occur, such as swabbing the surface of a print, albumen would behave as a brittle material because the albumen swells and shrinks so quickly. 4.3 STRESS-STRAIN BEHAVIOR OF THE ALBUMEN/PAPER COMPOSITEPaper is a felted network of fibers that are hydrogen bonded to each other. Contact with water disrupts these interfiber hydrogen bonds (Vitale 1992a). When unsized paper is immersed in the aqueous albumen solution, the interfiber bonds are disrupted. When water is removed, the albumen dries across the interfiber bonding sites. The result is a fiber-adhesive-fiber composite consisting of interfiber albumen “bonds.” However weak the albumen is, the albumen adhesive holding the interfiber sites together is still stronger than the equivalent made of hydrogen bonds. The strength of the fibers is many times stronger (10–20�) than the paper (Vitale 1992a); therefore the fibers are not failing at 10,340 psi. Thus, when the composite fails, the site of the failure is undoubtedly the interfiber albumen adhesive. The albumen/ paper composite is thus a unique material in the layered structure of the albumen print. 4.4 PROPOSED CAUSE OF CURLThe literature shows that differences in dimensional change and response to humidity cause curl (Smith 1950; Green 1983; Daniels and Fleming 1988). The albumen/paper composite swells and shrinks (fig. 6b) more than the paper support (fig. 6a). The composite is also significantly stronger (but not stiffer) than the paper or the albumen alone. It is probable, depending on the proportion of the composite layer in the cross section (fig. 8), that the composite controls the dimensional behavior of the print. If the composite layer expands more than the paper base, the print would be convex (albumen side out). On the other hand, during desorption from high humidity, shrinkage of the composite may be restrained by the paper base, resulting in a concave curl (albumen side in). In both cases, the mismatch in behavior of the layers builds stress as the print changes water content. Curl is a mechanism for relieving this stress. 4.5 DRIED-IN STRAINS IN ALBUMENIn light of literature on the effects of relative humidity on other protein systems, the loss of 1.25% in dimension (fig. 7) from randomly oriented fragments of albumen bars after one excursion to 85% RH, is not surprising (Calhoun and Leister 1959; Mecklenburg 1988; Karpowitz 1989). Calhoun and Leister (1959) found that when an unsupported Kodalith emulsion was taken above 80% RH, it shrunk 1.5%. They attribute this shrinkage to moisture-induced relaxation of dried-in strain. Because gelatin is a globular protein, unlike the linear collagen molecule, the one-time 1.25% reduction in length (under similar conditions) seen in albumen could be a related phenomenon. For albumen films, the source for dried-in strain seems apparent. Egg protein solids make up only 20% of the egg white; the remainder is water. When the denatured (disordered) hydrated globular protein solution dries down, the proteins are strained into a new shape dictated by the decrease in bulk. As bulk water evaporates from the native state, the solution is concentrated; when only associated water remains, the gel is dominated by hydrogen bonds between the water and proteins and within the proteins. At approximately 90% RH the material is approximately 28% water (29% for ovalbumin, 27% for lysozyme, Kuntz and Kauzmann, 1974). It is unclear that covalent cross-links exist in mechanically denatured egg albumen, but it is certain that hydrogen bonds dominate the solid material (Kuntz and Kauzmann 1974; Kinsella 1976). As the albumen solid is dried, either voids form in the place of the water or the globular molecules compact. The specific volume of dried albumen is only 3.5–7% greater than the solid components in the water swollen solid (Kuntz and Kauzmann 1974). This finding suggests that very few voids have been added to the solid during drying. Kuntz and Kauzmann (1974) argue effectively that given a probable void size (5�) and the surface-free energy of the voids, the formation of voids would be unfavorable. The energy (tens of Kcals) generated by the voids would be sufficient to change the conformation of the globular proteins severely. Thus, if voids formed, they must collapse as they form. In addition, x-ray diffraction analysis of dried bovine serum albumin (a related globular protein) indicates that there is less evidence of crystallinity in the dried state; thus, there is strong evidence that dried-in strains are created as the globular proteins are distorted when water is removed from the system (Kuntz and Kauzmann 1974). It is possible that the drying-induced molecular distortion further mechanically denatures the protein polymers, thereby rendering the distortions permanent. The behavior illustrated in figure 7 shows that some dried-in strains can be released. Reintroduction of water into the system disrupts hydrogen bonds within and between protein molecules. The potential energy created when the molecular distortions were formed is realized when water breaks the hydrogen bonds that secured the distortions in the dry state. That there is only one cycle of strain release suggests that some of the molecular-distortion becomes permanent. Several cycles of dimensional decrease would suggest that a form of metastable equilibrium was being reached at each rewetting and drying cycle. Further cycling would allow more potential energy to be released. 4.6 SHRINKAGE OF ALBUMEN AND PAPER COMPOSITESAlbumen and paper composites continue to shrink when rewet and dried. The one-time shrinkage of unsupported albumen observed in figure 7 should be released when albumen prints are rewet for sensitization, development, and washing after the initial dry-down of the albumen layer. However, historical albumen prints were found to shrink when rewet and dried in Messier and Vitale (1994), and the experimental The moisture vs. adsorption-desorption curves of the albumen and paper composites (figs. 6b, 6c, and 6d) exhibit discontinuous behavior of the swelling curve between 60% RH and 80% RH. This lack-of-swelling behavior suggests that (1) swelling stops for some reason, or (2) some form of shrinkage is occurring. Paper swells and shrinks no more that 1–2% when wet and dried. Albumen swells and shrinks 17% (or more) under the same conditions. The two albumen and paper composites in figure 5 swell 4% CD; thus the albumen is influencing the dimensional behavior of the paper base. Paper is very weak when wet; the cotton paper in this experiment has a tensile strength of 100 psi when wet (Vitale 1992a). It could be said that the albumen alters the paper and that the paper restricts the swelling of albumen in the x and y planes. It is assumed that the swelling not allowed in the xand y planes is translated into z direction (thickness) swelling. In the E-SEM work on the swelling of a historical albumen print (Messier and Vitale 1993), it was observed that the albumen swelled 5% in the x plane and 22% in the z plane when taken from 63% RH to immersion. The shrinkage of albumen prints and albumen/paper composites after successive rewetting is evidence that all the dried-in strains released in one swelling of unsupported albumen are not released when the albumen is attached to a paper support that will not swell much beyond 4–5%. It must be said that, while (1) the print shrinkage phenomena, (2) the 1.25% shrinkage of unsupported albumen when rewet and dried, and (3) the discontinuous behavior during swelling have not been correlated, their associations do offer a tantalizing provisional explanation for the continued shrinking of the albumen layer. 4.7 RATE OF SHRINKAGEThe stress-strain data for the unsupported albumen with pre-existing cracks (Blanquart-Evrard method) reported in table 1 was generated from experiments run at a rate of 1.5% stretch per 12 minutes. The data predict that a similar precracked albumen layer will fail after 1.5% stretch in 12 minutes or less. If a print swelled 5% during immersion and was dried between blotters for 40 minutes, the rate would be matched. Faster drying would presumably result in greater cracking. It was shown through the ultrasonic impediometry data that a faster rate increases the brittleness of the albumen. To calculate an appropriate drying time, the following points should be considered. Shrinkage is never linear. The linear rate must be increased a given percentage based on the material to correct for the nonlinearity. Drying is always slow at the end of the process because movement of water through a solid is generally slow. Following the major portion of the drying process the solid has compacted due to the water loss. The structure is less open. Thus movement of the water takes longer, slowing the rate of drying. For paper, the time would need to be increased by approximately 20% (Rance 1954 data taken with Sugarman and Vitale 1992). This value has not been calculated for albumen. If the nonlinearity is assumed to be 20% for albumen, the following calculations would result:
Thus, if the maximum shrinkage of a wet albumen print is on the order of 5%, then a 1 hour drying time would be sufficient for the The average performance for crack population increase (41%) and crack width increase (69%) would approximate those in Messier and Vitale (1994) because it was observed that the prints generally dried over a 1.5 � 0.5 hour period even though the historical prints stayed between blotters for 24 hours. |