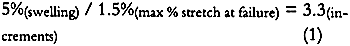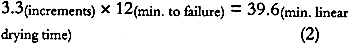PHYSICAL AND MECHANICAL PROPERTIES OF ALBUMEN PHOTOGRAPHSTIMOTHY VITALE, & PAUL MESSIER
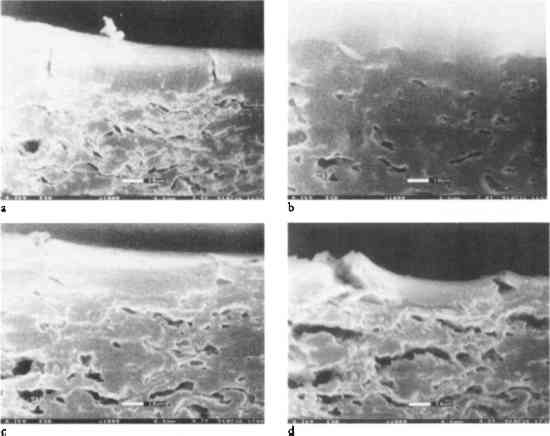
ABSTRACT—Fundamental physical and mechanical properties of albumen photographs were studied with the goal of explaining the tendency for pre-existing cracks in the albumen layer to increase in number and width when exposed to water. Samples were taken from the following materials: historical 19th-century albumen prints, unsized cotton paper, albumen prints made by the authors for the purpose of the study, albumen/paper composites, and “bars” made by casting albumen in silicone grease troughs. The samples were subjected to a variety of tests, including stress-strain analysis, ultrasonic impediometry, and measuring before, during, and after exposure to humidity. The various materials that make up an albumen photograph respond differently to changes in relative humidity, leading to problems between the components. Care should be taken when treating albumen prints with either water or water vapor, and the conditions under which the prints are stored should be carefully considered. 1 INTRODUCTIONIn a previous study (Messier and Vitale 1994), it was shown that all albumen prints exhibit cracking in the albumen layer. Aqueous treatment increases the number and width of these cracks and decreases the print gloss. In a study of the real-time behavior of albumen prints exposed to changes in relative humidity and water immersion using environmental electron scanning microscopy (E-SEM), similar behavior was observed (Messier and Vitale 1993). To explain this behavior, the evaluation of materials properties of albumen, an albumen and paper composite, and an experimental albumen print was undertaken. Figure 1 shows the dramatic effects of water on the albumen layer. This series of photo micrographs taken with an E-SEM shows a historical albumen print in cross section (Messier and Vitale 1993). The sample was placed in a grooved mount and not restrained in any way. The amount of water vapor (no other gas present) and the temperature of the sample were controlled within the E-SEM sample chamber. The sample was brought to 100% relative humidity and then immersed in water. Reversing the process, the sample was dried incrementally by decreasing the water vapor pressure in the sample chamber gradually to 14% relative humidity.
1.1 LITERATURE1.1.1 Egg Albumen
Messier (1991) reviewed the literature on the nature of egg albumen. The native albumen solution is a mixture of 13 globular proteins (Powrie and Nakai 1986). Of the six major polymeric proteins, ovalbumin is the largest constituent, 54% of native egg white. Egg white is approximately 88% water. Kinsella (1976) states that both mechanical denaturation and the introduction of salts will result in the availability of additional hydrophilic sites that were formerly within the protein globule. These sites increase the ability of the molecules to bind water. Bound water is attached through hydrogen bonds to polar side chains in lysine, glutamic acid, tyrosine, and similar amino acids, and to oxygen and nitrogen in the peptide bonds between individual amino acids (Bull 1944). In solution, the three-dimensional globular polymers have shells of tightly bound water surrounded by large quantities of associated water and massive quantities of bulk It is unclear if covalent cross-links exist in mechanically denatured egg albumen (Kuntz and Kauzmann 1974). Benesch and Benesch (1948), reviewing alcohol denaturation of egg albumen, report that denaturation seems to make sulfhydryl groups available. The primary Clovin (1964) and Putnam (1953) review the denaturation process. Putnam (1953) reports that egg albumen is denatured by the surface effects of frothing and that the egg albumen is the most susceptible of the proteins studied to denaturation by the action of coating. MacDonnell et al. (1950) show that mechanical shear (not pressure) results in denaturation, but that in solution the effects are partially reversed. Joly (1955) reports that mechanical denaturation can in some cases be partially reversed. Kauzmann and Simpson (1953) study the effects of chemical (urea) denaturation on the unfolding of the ovalbumin molecule due to disruption of hydrogen bonds. MacDonnell et al. (1955) studied the nature of single and multiple components of egg albumen that were both native and mechanically denatured. 1.1.2 Gelatin: A Related ProteinGelatin is a related globular protein. Bienkiewicz (1990) and von Endt and Baker (1991) review leather-water and gelatin systems. Harrington and Von Hippel (1961) review the structure of collagen and gelatin. Gelatin is a single helical molecule from the collagen triple helix. When separated in solution, gelatin takes a globular form. Literature on the effects of humidity changes on gelatin are of great value for interpreting the behavior of albumen. Calhoun and Leister (1959) studied the dimensional stability of an unsupported gelatin emulsion layer. They found that a unsupported Kodalith emulsion shrinks 0.7% between 70% and 80% RH, and they attribute this shrinkage to moisture-induced relaxation of dried-in strain. Mecklenburg (1991) reports that rabbit skin glue (gelatin) shrinks each time it is cycled to 95% after being taken to lower relative humidity. Karpowicz (1989) reports that rabbit skin glue, when dried with different types of restraint, will always shrink upon elevation to high humidity and return to normal humidity. He corroborates that glue continues to shrink when cycled (11 times) from high (83% and 91%) to moderate (54%) relative humidity. 1.1.3 Hardening of Albumen PrintsKlotz (1953) and Benesch and Benesch (1948) showed that silver strongly binds to functional groups, specifically sulfhydryl groups. Benesch and Benesch (1948) found that silver is complexed by crystalline egg albumen and cystine hydrochloride and suggested that denaturation makes more sulfhydryl groups available, the assumption being that cystine groups are the binding sites. Cystine is found in several protein polymers in egg albumen: ovalbumin, ovotransferrin (formerly conalbumin), ovo-mucoid, and lysozyme. However, in most of the protein present in albumen, the cystine is a complex of two amino acids joined with a disulfide bond. Free cystine is predominately available only in ovalbumin (one or two groups), which is the primary constituent (54%) of egg albumen.
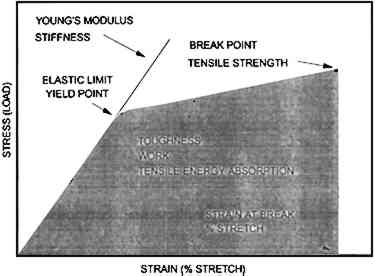
MacDonnell et al. (1955) and Cunningham (1976) determined that ovalbumin was the major component of drainage from frothed egg white. Drainage could be as high as 60–75%, depending on the method of frothing; multiple frothings yielding the highest percentage of ovalbumin. Lysozyme, ovotransferrin, ovomucoid, and ovomucin were partially decreased by drainage from froth. Cunningham (1976) also found that globulins A1 and A2 were totally removed in the froth. When denatured, ovalbumin can have up to four available sulfhydryl groups (Feeney 1964); though Cunningham Ovotransferrin is known to act similar to blood proteins that sequester metal ions. Ovotransferrin contains only double cystine groups linked by a disulfide bond and no free sulfhydryl groups (Powrie and Nakai 1986). Alderton et al. (1946) has shown that ovotransferrin has the in vivo biological function of sequestering di- and trivalent metal ions that interfere with activity of the egg. Windle et al. (1963) states that there is strong evidence that propitiously spaced pendant oxygen, nitrogen, and bicarbonate (groups) bind metal ions in ovotransferrin. Munson (1993) has confirmed that in modern albumen print making, sensitized albumen is not soluble in the wash water, whereas the presensitized, salted, and denatured albumen is soluble. Towler (1864) remarks that sensitized albumen prints are coagulated (i.e., “set”) by sensitization and that they are quite hard and require no varnish. The introduction of silver into albumen-coated paper makes the sensitized material somewhat insoluble, but permeable, to water. Based upon the literature, it appears that silver is bound both to (1) oxygen, nitrogen, and other electron-rich pendant groups in ovotransferrin, and to (2) free sulfhydryl groups in ovalbumin and other protein polymers if opened by mechanical denaturation. It is probable that favorably spaced pendant groups between protein molecules also bind metal ions. Towler (1864) reports that unsalted albumen will sensitize weakly when exposed (floated) on a silver nitrate bath; this property indicates that some silver binding capability exists in mechanically denatured egg albumen. It is not known if disulfide bonds form between the protein polymers as egg albumen dries. If disulfide cross-links are created between polymer chains during drying, this result would satisfy the traditional explanation for “hardening” a material, i.e., making it less soluble or insoluble. That fact that dried-down, denatured, and salted albumen remains soluble in water suggests that few if any new disulfide bonds form as a result of drying. The behavior reported by both Munson (1993) and Towler (1864) suggests that the introduction of silver creates some form of “cross-links” that “hardens” egg albumen. Since drying egg albumen does not result in insolubility, then the binding effect described by Windle et al. (1963) for ovotransferrin is a strong candidate for the “hardening” of albumen because the appropriate pendant groups exist in the albumen-coated paper when it is exposed to silver during sensitization. 2 EXPERIMENTAL DESIGN2.1 SAMPLE PREPARATION2.1.1 19th-Century PrintsThe three 19th-century albumen prints used in this study are typical examples of the mature albumen printing process used between the mid-1860s and the 1890s. They have a moderate to high sheen and appear to have been coated with albumen by large-scale, commercial manufacturers. Mounted prints samples were removed from mounts through aqueous immersion. They were thoroughly dried prior to testing. 2.1.2 Unsized Cotton Paper (Paper Base)The paper was an unsized sheet composed of 91% cotton fiber and 9% kraft and bleached wood pulp. It was a machine-made, wove paper. Six samples were used. 2.1.3 Experimental Albumen PrintsThe three prints for this study were made to simulate the 19th-century process. A cotton paper base was used unsized for the base of the experimental albumen prints and the albumen/paper composite. Although contrary to 19th-century practice, the unsized paper was used to focus the experiment more precisely on the interaction 2.1.4 Albumen/Paper CompositeThe three composites of paper and albumen were created by soaking the washed cotton paper for 30 minutes in a tray of the albumen. The saturated sheet was hung from a corner and allowed to air-dry for 2 days. 2.1.5 Unsupported Nonsensitized AlbumenTwo types of unsupported albumen “bars” were fabricated: five with internal microfissures and cracks using the Blanquart-Evrard method (Reilly 1980) and five crack-free using the Towler plate method (Towler 1864). These bars were approximately 0.2 in. wide and 0.05–0.1 in. thick. Casting albumen into a film or bars proved to be very difficult. As albumen dries, it shrinks massively and cracks in most situations. The drying process for the Blanquart-Evrard method albumen with 1.86 M NaCl (Reilly 1980) on a smooth polyester base shows the growth of salt crystals, which force crazing in the coating. Following crazing, large cracks formed in the sample. The Towler plate method with 0.06 M KI (Towler 1864) and multiple frothings, shows similar salt crystallization behavior. Drying the equivalent salt solutions (no proteins) produced crystal patterns related to the 0.06 M and 1.86 M salted albumen solutions. Albumen coatings on glass and unsupported bars made using the Towler plate method (1) with 0.06 M KI (Towler 1864) and (2) with no salt of any type show no crystal growth patterns and few cracks. The Towler plate method involves prolonged frothing (one cycle only) of the salted or unsalted albumen, placing all froth and liquid in a clear glass bowl and covering the surface of the foam with an unsized, thin paper cap cut to the circumference of the foam surface (paper touching edge of bowl). When the foam has shrunk from the liquid surface and the paper is crusty (approximately 2 days at 50% RH), the liquid can be carefully drained from the bowl. The coatings made on glass microscope slides can be made to crack massively if temperature and humidity are cycled or held at low relative humidity for prolonged periods. The crack-free albumen bars made using the Towler plate method remain stable under these same conditions. High shear frothing (3 minutes on low speed in a kitchen blender) using the Blanquart-Evrard method with 1.86 M NaCl salted albumen, with approximately 2 weeks of room temperature fermentation (inoculation by leaving outdoors), produced bars with microfissures The crack-free bars used for mechanical testing were made using the Towler plate method with 0.06 M KI (Towler 1864). The development of a casting method required considerable experimentation and persistence. During the drying process, if the shrinking albumen was in any way impeded, the sample would shatter. Casting was attempted using a Teflon mold. Results were very poor: all casts dried with severe cracking and were unusable. A bed of mercury was added to the Teflon mold: the albumen would not form into bars but formed misshapen islands on the mercury with the edges defined by the Teflon walls. Finally, molds were made by cutting troughs in silicone grease. This method allowed the albumen to shrink virtually unimpeded and the drying bar to deform its surroundings rather than meeting the resistance of rigid walls. The method also had the advantage that the dried samples could be carefully excavated from the molds by removing the surrounding grease. Residual grease was removed with petroleum benzine. It was impossible to sensitize the albumen bars without them breaking into chips. Upon wetting, the bars broke apart into chips of approximately the same size, 1–1.5 mm in diameter. 2.1.6 Unsupported, Sensitized AlbumenChips of sensitized Blanquart-Evrard method bars were used in the humidity and water swelling experiments. Aside from sensitization, the chips were toned, fixed, and washed as described above for the experimental albumen print. 2.2 MECHANICAL ANALYSIS2.2.1 Equilibrium Stress-Strain ProtocolTests were performed using a tensile strength test bed designed and built by Mecklenburg (1988). The paper-based samples were strained at a rate of 0.0025 in./minute. Unsupported albumen was strained at 0.00025 in./minute. The stepwise, slow rate of strain allows the load to be evenly distributed throughout all parts of the sample before measurements are taken and a new strain applied. Strain is applied, and the resulting stress is measured until the sample fails. This mechanical analysis protocol has been designated equilibrium stress-straining (Mecklenburg 1988). Measurements were taken at 20.5 � 0.5�C) and 50% (� 2%) relative humidity. Paper-based samples were measured in both the machine direction (MD) and cross-machine direction (CD). Generally three repetitions of each sample were made, with the exception of the paper base, which was repeated six times. Essential information generated by stress-strain data include: Young's modulus, tensile strength, yield point, percent stretch, and work. Figure 2 indicates the various mechanical properties on a typical stress-strain curve for an unsized cotton paper. The slope of the initial linear portion of the stress-strain curve is known as Young's modulus. This is a region of elastic behavior, where strain-induced changes are recoverable. When in this region, the material will return to its original size and shape when the load is removed. Young's modulus is a measure of a material's stiffness. The yield point is the point at which load begins to cause irreversible deformation and marks the transition between recoverable and permanent deformation. Strain builds until failure occurs. Percent stretch is the strain at failure, times 100. The stress at failure is the tensile strength. The total area under the stress-strain curve is an indication of a material's toughness. This area
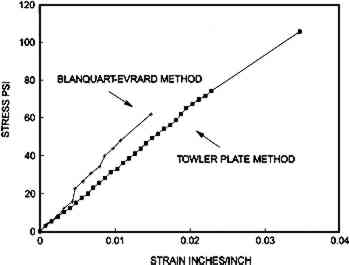
2.2.2 Ultrasonic ImpediometryTo determine the mechanical properties of unsupported albumen, ultrasonic impediometry was used, with the assistance of Donald Hunston, Polymers Division, National Institute of Standards and Technology. The first reflection from a 5 MHz quartz rod transducer was used to determine shear modulus. The face of the transducer was oriented so samples could dry flat on the face and the shock wave would travel through the thickness of the sample. The albumen was applied in solution and dried on the horizontal transducer face. Measurements were made continuously during drying. Only one run is reported because it was the only sample to reach completion intact. Three other runs were similar and confirmed the accuracy of the measurement. Initial runs showed that the shear modulus increases up to approximately 3,500,000 psi, and then the layer cracked. As the sample continued to dry past this halfway point, it peeled away from the surface and acoustic contact was lost, causing loss of signal. After some experimentation, a small piece of Kimwipe was dropped on the top surface of the drying albumen. This reinforcement did not affect the shear modulus of the albumen oriented normal to the transducer face. The fact that the intermediate shear modulus peak was 1,131,000 psi (Young's modulus = 2,640,000 psi) and well within the range of initial samples proved that adding reinforcement out of plane to the shock wave had no effect on the signal. The reinforcement did, however, prevent cracking and the loss of signal. The sample took approximately 30 minutes to dry. After peaking at approximately 15 minutes, the modulus decreased to a steady state in equilibrium with the room humidity, which was approximately 40% RH at approximately 21�C. Shear modulus is calculated using the equation E = 2G(1v), where E = Young's modulus, G = shear modulus, and v = Poisson's ratio and assuming a Poisson's ratio for albumen of 0.33 (based on similar figures for plastics and related protein polymers). Density of the native egg white (unsalted) was calculated to be 1.663 g/cc. 2.3 MEASUREMENT OF EXPANSION WHEN WETAll samples were immersed in water and had their in-plane elongation measured. The three examples of each modern sample were measured three times each. Five historical albumen prints were measured three times. Once thoroughly wetted, the paper-based samples were removed from the deionized water and quickly measured. Nine individual unsupported albumen fragments were measured three times each. They had a tendency to dissolve when fully immersed in water. Therefore, they were wet and measured in situ under a Leitz component measuring microscope. Measurements were taken within 30 seconds of exposure to water; thereafter the samples swelled into a shape that could not be measured accurately. 2.4 MOISTURE SORPTION-DESORPTION BEHAVIOR OF ALBUMEN PRINTS AND COMPONENTSPaper-based samples were placed in a sealed humidity chamber. Three samples of each material were measured three times at each stage; the entire experiment (1 month) was not repeated due to time constraints. The relative humidity was precisely controlled through the use of a General Eastern dew point hygrometer and a custom-made apparatus designed by Timothy Padfield, formerly of the Conservation Analytical Laboratory, Smithsonian Institution. The hygrometer controlled the amount of moisture added or withheld until the desired relative humidity was achieved. Conditions were maintained through continuous monitoring. Conditioning time at each relative humidity measurement point was 24 hours. Temperature was controlled externally and remained at 20�C � 0.5�. The samples were measured to the nearest 0.01 in. 2.5 SORPTION-DESORPTION BEHAVIOR OF UNSUPPORTED SENSITIZED ALBUMENTen unsupported, sensitized, randomly oriented albumen chips were placed on a rigid support. The chips were measured in length and width five times each. A clear glass Petri dish was inverted over the samples. Silicone grease was used to create an airtight seal between the base and the inverted Petri dish. Small dishes of saturated salt solutions were placed in the chamber. The various salts had the effect of conditioning the air within the sealed chamber to specific relative humidities. Since the Petri dish was transparent, measurements could be made with the chamber sealed. The albumen fragments were measured with the Leitz component-measuring microscope. The fragments were measured first at 50% RH, because those were the conditions in which they were dried. Temperature remained at 20�C � 0.5�. Twenty albumen fragments were used for this experiment. Conditioning time at each relative humidity was 24 hours. The average dimensional change for these twenty fragments was calculated for each measurement point in the relative humidity cycle. Swelling and shrinkage were highly variable. A few samples showed a maximum change of 30%, while others showed a change of approximately 10%. The experiment was repeated three times to confirm the dramatic results and the variability of the change. 3 RESULTS3.1 MECHANICAL ANALYSIS3.1.1 Equilibrium Stress-Strain of Unsupported Nonsensitized AlbumenAlbumen is soft with a modulus of 3,031–4,166 psi, moderately extensible with a 1.5–3.4% stretch, and very weak with a tensile strength of 62–105 psi. The albumen with microfissures and cracks is only slightly weaker than the crack-free albumen. Figure 3 shows averaged stress-strain curves from multiple individual unsupported albumen samples measured using the equilibrium stress-strain protocol, which features slow strain rate. The mechanical data are listed in table 1.
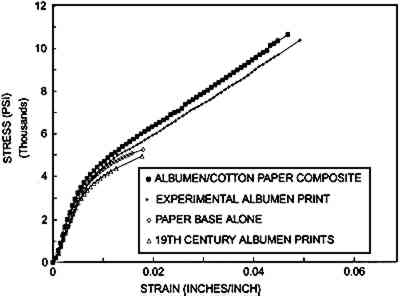
MECHANICAL PROPERTIES OF ALBUMEN PRINTS AND COMPONENTS Unexpectedly, the load at break and modulus for the two different samples (cracked and crack-free) is similar. The mechanical data for samples containing numerous small fissures were gathered from the Blanquart-Evrard method bars. Mechanical data gathered from the crack-free samples used the Towler plate method bars. The principal difference is that the crack-free bars stretch 3.4% prior to failure, while the precracked samples could only be stretched 1.5% before failure. 3.1.2 Physical Manipulation of Unsupported Nonsensitized AlbumenAlbumen is a light, lemon-colored, clear material. Upon handling, most observers 3.1.3 Ultrasonic Impediometry of Unsupported Nonsensitized AlbumenUsing 5 MHz ultrasonic impediometry to determine the shear modulus of Blanquart-Evrard method albumen, it was found that very high rates of strain increase stiffness, resulting in a Young's modulus of 1,270,000 psi (shear modulus = 477,000 psi) of the dry albumen. Although it is difficult to compare straining rates, 5 MHz ultrasonic impediometry (dynamic mechanical technique) is approximately 10 orders of magnitude faster than the equilibrium stress-strain protocol. Only one complete run is evaluated here, but three similar partial runs confirmed the magnitude of the modulus. 3.1.4 Equilibrium Stress-Strain of Prints, Paper, and CompositeFigure 4 shows a comparison of three averaged mechanical analyses using the equilibrium stress-strain protocol for the (1) three different 19th-century albumen photographs (one sample from each), (2) the unsized cotton paper, (3) the albumen print made for this experiment, and (4) albumen/paper composite.
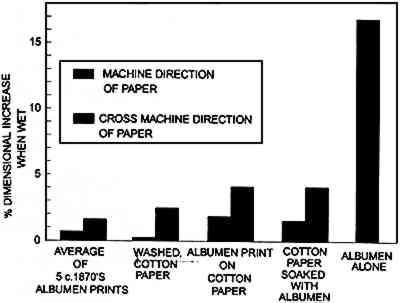
From figure 4 it is evident that the curves for the uncoated paper base and the curves for the experimental albumen prints are dissimilar and that the experimental print is almost identical to the albumen/paper composite. The moduli of all of the samples are quite close, within 25%. The principal difference between the plots is that the paper base cannot carry a load beyond 5,220 psi. The addition of albumen to both the composite and the experimental print increases the ability of the paper base to stretch and carry a load. For the purpose of comparison: single cotton fibers stretch 20% and rupture at 50,000–100,000 psi (Vitale 1992a). The tensile strength of the experimental albumen print and that of the historical sample are very different, while their moduli are the same. The historical print behaves similarly to the uncoated cotton paper. The behavior of the albumen/paper composite made with nonsensitized albumen is almost identical to the experimental print. The albumen layer on the albumen print was sensitized. It appears that sensitization and the related “hardening” of the albumen component do not influence mechanical behavior significantly. 3.2 SWELLING WHEN WETFigure 5 shows the results of swelling in water. Unsupported albumen swells more than 17% when exposed to water; swelling above 17% could not be measured accurately. This behavior is designated by reporting the swelling as 17%+. Albumen prints show different behavior. The five historical samples swelled an average of less than 1% in the machine direction (MD) and just under 2% in the cross-machine direction (CD). The experimental print swelled nearly 2% MD and 4% CD. The albumen/paper composite had virtually the same response to water. The washed cotton paper had an increase of less than 0.5% MD and an increase of 2.75% CD. The differences between samples are obviously due to the addition of albumen to the paper. As with the mechanical testing results, the behavior of the composite samples is not dominated by the properties of either the paper base or by albumen, but by a combination of both.
3.3 MOISTURE SORPTION-DESORPTION BEHAVIOR OF ALBUMEN PRINTS AND COMPONENTS3.3.1 Size Changes
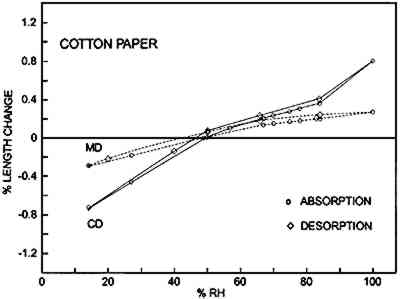
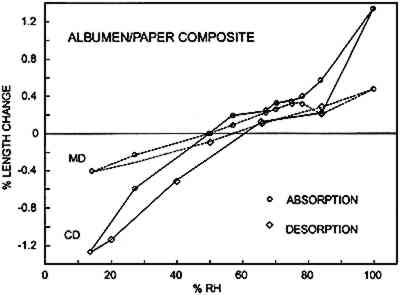
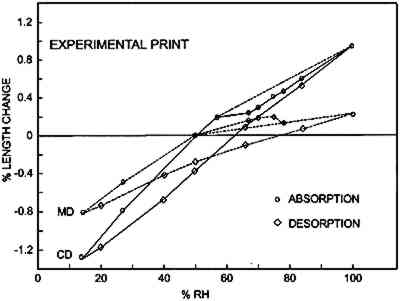
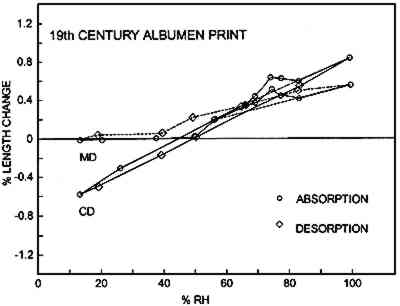
Figures 6a–d show the sorption-desorption behavior for all samples that are paper or have a paper base; three samples of each were
Table 2 is a summary of the results for this experiment. The values for the shrinkage of the experimental albumen print (n = 3) compare favorably to results of previous study (Messier and Vitale 1994), which shows that a group of 20 historical albumen prints shrank 0.19% MD and 0.35% CD. TABLE 2 AVERAGE DIMENSIONAL CHANGES MEASURED AT 50% RH AFTERDESORPTION FROM 100% RH 3.3.2 Moisture vs. Sorption-Desorption CurvesThe unsized cotton paper (fig. 6a) displays normal hysteresis behavior. It changes 0.5% MD and 1.6% CD. The albumen/paper composite (fig. 6b) displays very different behavior even though it is made using the same paper. At about 60% RH the CD curve shows that the sample stops increasing in length and does not resume normal behavior until approximately 80% RH. The behavior of the MD curve is also quite odd; it shows a shrinkage between 78% RH and 84% RH. The experimental albumen print (fig. 6c) also displays the odd behavior seen for the albumen/paper composite samples. In CD, the sample appears to stop increasing in length between 56% RH and 70% RH. The MD sample shrinks slightly between 75% RH and 78% RH. This type of discontinuous behavior is also exhibited in the adsorption phase of a single historical print (fig. 6d).
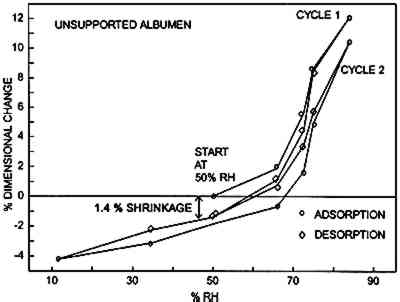
3.4 SORPTION-DESORPTION BEHAVIOR OF UNSUPPORTED, NONSENSITIZED ALBUMENThere is average 2% dimensional increase in the 10 unsupported, nonsensitized albumen chips when the relative humidity is increased
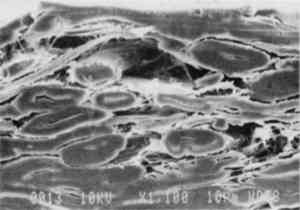
The albumen shrank 1.25% after one excursion from 50% RH to 85% RH and back to the initial RH 50%. On the second cycle the shrinkage did not occur. 3.5 EVIDENCE OF AN ALBUMEN/PAPER COMPOSITEFigure 8 is a scanning electron microscope micrograph showing a 19th-century albumen print in cross section. Note that a thin layer of uppermost paper fibers is fully permeated with albumen. Sizing appears to have restricted the penetration of liquid albumen during the albumen-coating process.
4 DISCUSSION4.1 SOURCE OF ALBUMEN CRACKING4.1.1 Propagation of Albumen CracksAll albumen prints examined by the authors, including the experimental albumen print, were observed to be cracked. Thus, the mechanical properties used to predict the behavior of albumen prints are those from the bars with pre-existing cracks. Figure 3 shows that unsupported nonsensitized albumen with pre-existing cracks can be strained only 1.5% before failure occurs and that the tensile strength is a very low 62 psi. Figure 7 shows that unsupported sensitized albumen swells 12% when the humidity is increased from 50% RH to 85% RH, and 17%+ when immersed. Figure 5 shows the behavior of albumen on paper: the historical albumen prints swelled 1.7% (CD) when wet. When these materials shrink, the strain limit of the albumen will be exceeded, and cracking will result. It is important to note that the bars were nonsensitized albumen, while the prints are sensitized albumen. Substantial evidence exists that albumen is physically hardened and rendered somewhat insoluble by sensitization. While it is not possible to test directly, the effect of hardening on mechanical properties may not be significant because the adsorption-desorption behavior of the sensitized experimental albumen print is not markedly different from that of the nonsensitized albumen/paper composite. 4.1.2 Direction of CrackingAll albumen prints have cracks. Even modern commercial (Chicago Albumen Works) unsensitized albumen papers exhibit cracking (Messier 1991). It was observed in the treatment-based experiment (Messier and Vitale 1994) that 14 of 20 historical albumen prints had a predominant crack pattern that ran along the machine direction of the paper. Since cracks propagate 90� to applied stress, it can be concluded that the albumen layer experiences greater stress in the cross-machine direction of the paper base. A possible explanation for this observation can be found in the sorption-desorption data for unsupported sensitized albumen (fig. 7). During the desorption phase of the print (fig. 6a), the paper base is slightly more expanded in 4.2 RATE-OF-STRAIN–DEPENDENT PROPERTIES OF UNSUPPORTED NONSENSITIZED ALBUMENThe differences in moduli caused by differences in strain rate, combined with observations based on physical handling of the material, indicate that albumen has pronounced rate-of-strain–dependent properties. In the fast strain rate domain it is brittle, and in the slow domain it is rubbery. Under common museum conditions where humidity or water content change slowly, cracked albumen acts like rubber, offering low resistance to stress or strain and yielding a normal amount before failure. When sudden changes occur, such as swabbing the surface of a print, albumen would behave as a brittle material because the albumen swells and shrinks so quickly. 4.3 STRESS-STRAIN BEHAVIOR OF THE ALBUMEN/PAPER COMPOSITEPaper is a felted network of fibers that are hydrogen bonded to each other. Contact with water disrupts these interfiber hydrogen bonds (Vitale 1992a). When unsized paper is immersed in the aqueous albumen solution, the interfiber bonds are disrupted. When water is removed, the albumen dries across the interfiber bonding sites. The result is a fiber-adhesive-fiber composite consisting of interfiber albumen “bonds.” However weak the albumen is, the albumen adhesive holding the interfiber sites together is still stronger than the equivalent made of hydrogen bonds. The strength of the fibers is many times stronger (10–20�) than the paper (Vitale 1992a); therefore the fibers are not failing at 10,340 psi. Thus, when the composite fails, the site of the failure is undoubtedly the interfiber albumen adhesive. The albumen/ paper composite is thus a unique material in the layered structure of the albumen print. 4.4 PROPOSED CAUSE OF CURLThe literature shows that differences in dimensional change and response to humidity cause curl (Smith 1950; Green 1983; Daniels and Fleming 1988). The albumen/paper composite swells and shrinks (fig. 6b) more than the paper support (fig. 6a). The composite is also significantly stronger (but not stiffer) than the paper or the albumen alone. It is probable, depending on the proportion of the composite layer in the cross section (fig. 8), that the composite controls the dimensional behavior of the print. If the composite layer expands more than the paper base, the print would be convex (albumen side out). On the other hand, during desorption from high humidity, shrinkage of the composite may be restrained by the paper base, resulting in a concave curl (albumen side in). In both cases, the mismatch in behavior of the layers builds stress as the print changes water content. Curl is a mechanism for relieving this stress. 4.5 DRIED-IN STRAINS IN ALBUMENIn light of literature on the effects of relative humidity on other protein systems, the loss of 1.25% in dimension (fig. 7) from randomly oriented fragments of albumen bars after one excursion to 85% RH, is not surprising (Calhoun and Leister 1959; Mecklenburg 1988; Karpowitz 1989). Calhoun and Leister (1959) found that when an unsupported Kodalith emulsion was taken above 80% RH, it shrunk 1.5%. They attribute this shrinkage to moisture-induced relaxation of dried-in strain. Because gelatin is a globular protein, unlike the linear collagen molecule, the one-time 1.25% reduction in length (under similar conditions) seen in albumen could be a related phenomenon. For albumen films, the source for dried-in strain seems apparent. Egg protein solids make up only 20% of the egg white; the remainder is water. When the denatured (disordered) hydrated globular protein solution dries down, the proteins are strained into a new shape dictated by the decrease in bulk. As bulk water evaporates from the native state, the solution is concentrated; when only associated water remains, the gel is dominated by hydrogen bonds between the water and proteins and within the proteins. At approximately 90% RH the material is approximately 28% water (29% for ovalbumin, 27% for lysozyme, Kuntz and Kauzmann, 1974). It is unclear that covalent cross-links exist in mechanically denatured egg albumen, but it is certain that hydrogen bonds dominate the solid material (Kuntz and Kauzmann 1974; Kinsella 1976). As the albumen solid is dried, either voids form in the place of the water or the globular molecules compact. The specific volume of dried albumen is only 3.5–7% greater than the solid components in the water swollen solid (Kuntz and Kauzmann 1974). This finding suggests that very few voids have been added to the solid during drying. Kuntz and Kauzmann (1974) argue effectively that given a probable void size (5�) and the surface-free energy of the voids, the formation of voids would be unfavorable. The energy (tens of Kcals) generated by the voids would be sufficient to change the conformation of the globular proteins severely. Thus, if voids formed, they must collapse as they form. In addition, x-ray diffraction analysis of dried bovine serum albumin (a related globular protein) indicates that there is less evidence of crystallinity in the dried state; thus, there is strong evidence that dried-in strains are created as the globular proteins are distorted when water is removed from the system (Kuntz and Kauzmann 1974). It is possible that the drying-induced molecular distortion further mechanically denatures the protein polymers, thereby rendering the distortions permanent. The behavior illustrated in figure 7 shows that some dried-in strains can be released. Reintroduction of water into the system disrupts hydrogen bonds within and between protein molecules. The potential energy created when the molecular distortions were formed is realized when water breaks the hydrogen bonds that secured the distortions in the dry state. That there is only one cycle of strain release suggests that some of the molecular-distortion becomes permanent. Several cycles of dimensional decrease would suggest that a form of metastable equilibrium was being reached at each rewetting and drying cycle. Further cycling would allow more potential energy to be released. 4.6 SHRINKAGE OF ALBUMEN AND PAPER COMPOSITESAlbumen and paper composites continue to shrink when rewet and dried. The one-time shrinkage of unsupported albumen observed in figure 7 should be released when albumen prints are rewet for sensitization, development, and washing after the initial dry-down of the albumen layer. However, historical albumen prints were found to shrink when rewet and dried in Messier and Vitale (1994), and the experimental The moisture vs. adsorption-desorption curves of the albumen and paper composites (figs. 6b, 6c, and 6d) exhibit discontinuous behavior of the swelling curve between 60% RH and 80% RH. This lack-of-swelling behavior suggests that (1) swelling stops for some reason, or (2) some form of shrinkage is occurring. Paper swells and shrinks no more that 1–2% when wet and dried. Albumen swells and shrinks 17% (or more) under the same conditions. The two albumen and paper composites in figure 5 swell 4% CD; thus the albumen is influencing the dimensional behavior of the paper base. Paper is very weak when wet; the cotton paper in this experiment has a tensile strength of 100 psi when wet (Vitale 1992a). It could be said that the albumen alters the paper and that the paper restricts the swelling of albumen in the x and y planes. It is assumed that the swelling not allowed in the xand y planes is translated into z direction (thickness) swelling. In the E-SEM work on the swelling of a historical albumen print (Messier and Vitale 1993), it was observed that the albumen swelled 5% in the x plane and 22% in the z plane when taken from 63% RH to immersion. The shrinkage of albumen prints and albumen/paper composites after successive rewetting is evidence that all the dried-in strains released in one swelling of unsupported albumen are not released when the albumen is attached to a paper support that will not swell much beyond 4–5%. It must be said that, while (1) the print shrinkage phenomena, (2) the 1.25% shrinkage of unsupported albumen when rewet and dried, and (3) the discontinuous behavior during swelling have not been correlated, their associations do offer a tantalizing provisional explanation for the continued shrinking of the albumen layer. 4.7 RATE OF SHRINKAGEThe stress-strain data for the unsupported albumen with pre-existing cracks (Blanquart-Evrard method) reported in table 1 was generated from experiments run at a rate of 1.5% stretch per 12 minutes. The data predict that a similar precracked albumen layer will fail after 1.5% stretch in 12 minutes or less. If a print swelled 5% during immersion and was dried between blotters for 40 minutes, the rate would be matched. Faster drying would presumably result in greater cracking. It was shown through the ultrasonic impediometry data that a faster rate increases the brittleness of the albumen. To calculate an appropriate drying time, the following points should be considered. Shrinkage is never linear. The linear rate must be increased a given percentage based on the material to correct for the nonlinearity. Drying is always slow at the end of the process because movement of water through a solid is generally slow. Following the major portion of the drying process the solid has compacted due to the water loss. The structure is less open. Thus movement of the water takes longer, slowing the rate of drying. For paper, the time would need to be increased by approximately 20% (Rance 1954 data taken with Sugarman and Vitale 1992). This value has not been calculated for albumen. If the nonlinearity is assumed to be 20% for albumen, the following calculations would result:
Thus, if the maximum shrinkage of a wet albumen print is on the order of 5%, then a 1 hour drying time would be sufficient for the The average performance for crack population increase (41%) and crack width increase (69%) would approximate those in Messier and Vitale (1994) because it was observed that the prints generally dried over a 1.5 � 0.5 hour period even though the historical prints stayed between blotters for 24 hours. 5 SUMMARY OF MECHANICAL AND PHYSICAL RESULTSThe mechanical and physical evaluations of albumen prints and albumen print components reveal the following:
6 CONCLUSIONS6.1 GENERALExcursions to high relative humidity and wetting swell the albumen more than it can stretch when it is dried. When it shrinks during desorption, its inherent low tensile strength results in stress relief cracking of the albumen. A layered structure in albumen prints and a mismatch in dimensional response of the layers to water vapor result in albumen print curl and planar distortions. The albumen/paper composite is stronger than the print, and it swells and shrinks more than the paper alone. At high rates of dimensional change, albumen behaves as a stiff brittle solid. At a slow rate of strain, on the order of 1.5% stretch in 12 minutes, it behaves as a rubber. Thus, surface swabbing with water and large climatic changes have the same ultimate result, even though the step-by-step actions are different. 6.2 STORAGE CONDITIONSThe mechanical and dimensional behavior of albumen photographs underscores the importance of a controlled storage environment with constant, moderate RH. A 30% or greater change in RH centered on 50% RH (between 35% and 65% RH) will exceed the strain limits of an unsupported albumen film at slow rates of change and will result in crack width increase or the introduction of new cracks. Parenthetically, 50% RH has no storage-specific importance; 6.3 TREATMENTThe materials properties findings have implications for the conservation treatment of albumen photographs. Water vapor treatments exceeding 30% (50 � 15% RH) and the direct application of liquid water result in swelling and shrinkage that will cause stress-release cracking. The most damaging stage of the wetting and drying process is the shrinkage during drying. During wetting the materials are plasticized and can yield to the forces of compression. In the case of the historical albumen print in the E-SEM experiment (Messier and Vitale 1993), the albumen layer should have swelled 17%+ in all directions, but it was restrained in the x and y planes. It swelled 5% and then shrank roughly 9%. Thus additional damage occurred during drying. In addition, the curling in the albumen layer occurred during the drying cycle. Rate of drying can influence crack population increase and crack propagation behavior. Drying an albumen print, after it has been immersed, usually occurs over 1 or more hours. For a print that swells 5%, an increase in crack population and crack propagation will occur because, no matter what the rate of shrinkage, cracking will result because the swelling is greater than 1.5%. If the same print is dried from immersion in less than 48 minutes, more cracks and greater crack width increase than those reported in Messier and Vitale (1994) should be observed because of the pronounced rate-of-strain dependency of the material. Slowing the drying time, by prewetting the blotter for example, may have no effects on crack width or crack population, but certainly there would be no detrimental effects due to a faster rate of shrinkage in this rate-of-strain–dependent material. 6.4 NEED FOR UNMOUNTING OR MOUNTINGThe long-term mechanical and physical effects of mounting albumen prints onto rigid supports need investigation. Is a mounted albumen photograph better preserved (in terms of the stability of the albumen layer) if the print is left on its mount? Would dry removal of a mount be preferable, or would the mechanical action also result in additional cracks in the albumen? The rate of strain during mechanical removal would, in most cases, surely be in the fast domain. Would albumen photographs that have never been mounted be more stable if they were adhered (using nonaqueous adhesive) to a rigid mount? If an albumen print is removed from its mount during the course of a treatment, is it better to return it to a rigid support, or should it remain unmounted? ACKNOWLEDGEMENTSThe authors wish to thank the following for their assistance in preparing this work. Duane Chartier, private conservation treatment and conservation science practice in Los Angeles, offered valuable assistance in uncovering the importance of rate-of-strain on albumen—an excellent resource. Donald Hunston, Polymers Division, National Institute of Standards and Technology, provided the use of ultrasonic impediometry equipment and interpretation of the data. Marion Mecklenburg, assistant director for conservation research at the Conservation Analytical Laboratory (CAL), Smithsonian Institution, provided insightful guidance, read the manuscript, and made many valuable contributions. Mark McCormick-Goodhart, photographic scientist, CAL, offered valuable advice and insight at many points in the project. Noreen Tuross, research biochemist, CAL, performed amino acid assay on two samples of albumen, before and after denaturing. Carol Grissom, chief of objects conservation, CAL, REFERENCESAlderton, G., W. H.Ward, and H. L.Fevold. 1946. Identification of the bacteria-inhibiting, iron-binding protein of egg white conalbumin. Archives of Biochemistry11: 9–13. Benesch, R., and R. E.Benesch. 1948. Ampero-metric titration of sulfhydryl groups in amino acids and proteins. Archives of Biochemistry19: 35–45. Bergquist, D. H.1986. Egg dehydration. In Egg science and technology, 3d ed.Westport, Conn.: AVI Publishing Co.285–323. Berlin, E., P. G.Kliman, and M. J.Pallansch. 1970. Changes in state of water in proteinaceous systems. Journal of Colloid and Interface Science31(4): 488–94. Bienkiewicz, K. J.1990. Leather—water: A system?Journal of the American Leather Chemistry Association85(10): 305–25. Bull, H. B.1944. Adsorption of water vapor by proteins. Journal of the American Chemistry Society66: 1499–507. Calhoun, J., and A.Leister. 1959. Effect of gelatin layers on the dimensional stability of photographic film. Photographic Science and Engineering3: 8–17. Clovin, R. J.1964. Denaturation: A requiem. In Proteins and their reactions. Westport, Conn.: AVI Publishing Co.69–83. Cunningham, F. E.1976. Properties of egg white drainage. Poultry Science55: 738–43. Daniels, V., and L. E.Fleming. 1988. Unpublished. London: British Museum, Conservation Science Division. Feeney, R. E.1964. Egg proteins. In Proteins and their reactions. Westport, Conn.: AVI Publishing Co.209–24. Green, C. J.1983. Curl, expansivity and dimensional stability. In Handbook of physical and mechanical testing of paper and paperboard. 2: 415–43. Harrington, W. F., and P. F.VonHippel. 1961. The structure of collagen and gelatin. In Advances in protein chemistry, vol. 16, ed.C. B.Anfinsen et al. New York: Academic Press. Joly, M.1955. Recent studies on the reversible denaturation of proteins. In Progress in biophysics and biophysical chemistry, vol. 5, ed.J.A.V.Butler and J. T.Randall. London: Pergamon Press. 169–221. Jones, R. T.1964. The structure of proteins. In Proteins and their reactions. Westport, Conn.: AVI Publishing Co.33–56. Karpowitz, A.1989. In-plane deformations of films of size on paintings in the glass transition region. Studies in Conservation34: 67–74. Kauzmann, W., and R. B.Simpson. 1953. The kinetics of protein denaturation. Journal of the American Chemical Society75: 5139–52. Kinsella, J. E.1976. Functional properties of proteins in foods: A survey. In CRC critical reviews in food technology. Cleveland: CRC Press. 219–80. Koltz, I. M.1953. Protein interactions. In The proteins: Chemistry, biological activity and methods, vol. 1, pt. b. New York: Academic Press. 727–806. KuntzJr., I. D., and W.Kauzmann. 1974. Hydration of proteins and polypeptides. In Advances in protein chemistry, vol. 28, ed.C. B.Anfinsen et al. New York: Academic Press. MacDonnell, L. R., R. E.Feeney, H. L.Hanson, A.Campbell, and T. F.Sugihara. 1955. The functional properties of the egg white proteins. Food Technology9(Feb.): 49–53. MacDonnell, L. R., H. L.Hanson, R. B.Silva, H.Lineweaver, and R. E.Feeney. 1950. Shear—not pressure—harms egg white. Food Industries22(2): 85–88.
Mecklenburg, M. F.1988. The effects of atmospheric moisture on the mechanical properties of collagen under equilibrium conditions. AIC preprints.American Institute for Conservation 16th annual meeting, New Orleans. Washington, D.C.: AIC. 231–44. Mecklenburg, M. F.1991. Some mechanical and physical properties of gilding gesso. In Gilded wood, ed.D.Bigelow. Madison, Conn.: Sound View Press. 163–70. Messier, P.1991. Work in progress: An analysis of the effect of water on the cracking of albumen photographs. In Topics in photographic preservation, vol. 4. Washington, D.C.: American Institute for Conservation Photographic Materials Group. 170–78. Messier, P., and T.Vitale. 1993. Cracking in albumen photographs: An ESEM investigation. Journal of Microscopy Research and Technique25: 374–83. Messier, P., and T.Vitale. 1994. Effects of aqueous treatment on albumen photographs. Journal of the American Institute for Conservation33: 257–78. Munson, D.1993. Personal communication. Chicago Albumen Works, Housatonic, Mass. Powrie, W. D., and S.Nakai. 1986. The chemistry of eggs and egg products. In Egg science and technology, 3d ed.Westport, Conn: AVI Publishing Co.97–139. Putnam, F. W.1953. Protein denaturation. In The proteins: Chemistry, biological activity and methods, vol. 1, pt. b. New York: Academic Press. 807–92. Rance, H. F.1954. Effects of water removal on sheet properties: The water evaporation phase. Tappi37(12): 640–52. Reilly, J.1980. The albumen and salted paper book. Rochester: Light Impression Corporation. Smith, S. F.1950. Dried-in strains in paper sheets and the relation to curling, cockling and other phenomena. Paper-Maker and British Paper Trade Journal. 119: 185–92. Stadelman, W. J., and O. J.Cotterill. 1986. Egg science and technology, 3d ed.Westport, Conn.: AVI Publishing Co. Sugarman, J. E., and T. J.Vitale. 1992. Observations on the drying of paper: Five drying methods and the drying process. Journal of the American Institute for Conservation31: 175–97. Timasheff, S. N.1964. The nature of interactions in proteins derived from milk. In Proteins and their reactions. Westport, Conn.: AVI Publishing Co.179–208. Towler, J.1864. The silver sunbeam: A practical and theoretical text book on sun drawing and photographic printing. New York: J. H. Ladd. VanKleef, F.1986. Thermally induced protein gelation: Gelation and rheological characterization of highly concentrated ovalbumin and soybean protein gels. Biopolymers25: 31–59. Vitale, T. J.1992a. Effects of drying on the mechanical properties of paper and their relationship to the treatment of paper. In Materials issues in art and archaeology, III, Materials Research Society Symposium Proceedings 267, ed.P. B.Vandiver et al. Pittsburgh: Materials Research Society. 429–45. Vitale, T. J.1992b. Effects of water on the mechanical properties of paper and their relationship to the treatment of paper. In Materials issues in art and archaeology, III, Materials Research Society Symposium Proceedings 267, ed.P. B.Vandiver et al. Pittsburgh: Materials Research Society. 397–427. vonEndt, D. W., and M. T.Baker. 1991. The chemistry of filled animal glue systems. In Gilded wood, ed.D.Bigelow. Madison, Conn.: Sound View Press. 155–62. Wall, J. S.1964. Cereal proteins. In Proteins and their reactions. Westport, Conn.: AVI Publishing Co.315–41. Windle, J. J., A. K.Wiersema, J.R.Clark, and R. E.Feeney. 1963. Iron and copper complexes of avian conalbumins and human transferrins by electron paramagnetic response. Biochemistry2(6): 1341–45. AUTHOR INFORMATIONTIMOTHY VITALE is currently a conservation consultant working from Aptos, California. He has a B.A. in art history from San Jose State University, 1973, and a M.S. in art conservation from the University of Delaware/Winterthur Art Conservation Program, 1977. He was a staff member at the Stanford University Museum, 1970–74, and served an apprenticeship with Sella Petri, a book conservator in San Francisco. He was head of paper conservation, Intermuseum Conservation Association, Oberlin, Ohio, 1978–82, and chief of the preservation branch, National Archives and Records Service, Washington, D.C., 1982–83. From 1983 to 1993 he worked at the Conservation Analytical Laboratory. PAUL MESSIER is a conservator in private practice working in the Boston metropolitan area. He has an A. B. in art history from Vassar College, 1984, and an M.A. and C.A.S. in the conservation of works of art on paper from State University College at Buffalo. He worked as an apprentice in photographic materials conservation with Jose Orraca from 1985 to 1987. From 1989 to 1990 he completed an intern-ship with Anne Cartier-Bresson at the Atelier de Restauration des Photographies in Paris. He worked as a postgraduate fellow with Timothy Vitale at the Conservation Analytical Laboratory of the Smithsonian Institution where he completed research into the effect of aqueous treatment of albumen photographs in 1991. From 1991 to 1994 he was head of the paper and photographic materials conservation department at the Rocky Mountain Conservation Center in Denver. As an adjunct faculty member within the art history department at the University of Denver, he developed and taught a course designed to introduce the field of art conservation to graduate and undergraduate students. Address: 77 Griggs Rd., Brookline, Mass. 02146.
 Section Index Section Index |