ASPECTS OF CHEMICAL RESEARCH IN CONSERVATION: THE DETERIORATION PROCESSROBERT L. FELLER
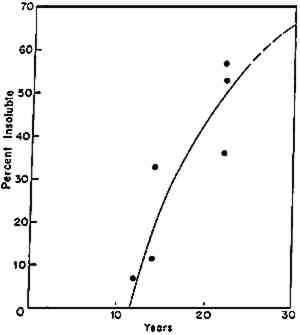
5 INDUCTION-TIME BEHAVIOROf considerable interest is the phenomenon of induction time (fig. 2d): a “time during which no observable change occurs in a chemical reaction or in a physical property, subsequently followed by such a change” (Alger 1989, 46). If conservators observe the condition of an artifact during an induction period and this period is a feature of its particular aging behavior, they may be lulled into thinking that changes are not occurring and that the artifact is in no imminent danger of change, only to find, a relatively short time thereafter, that decided changes have become apparent. Thus, as an initial reaction to a frustrating problem, Calmes (1993, 98) has been led to say of cellulose nitrate: “There appears to be no intermediate stage between the time when the film is in good condition and the time when it is obviously deteriorated.” We now know that research has been able to detect a number of intermediate stages. One of the earliest examples of induction time that the author encountered is in the cross-linking of butyl methacrylate polymers. When the data reported many years ago are considered (fig. 3), one sees that there is an apparent induction period of 11 years before insoluble (cross-linked) matter was formed in the various butyl methacrylate–based coatings that had been exposed under skylights on the wall of the laboratory of the Fogg Art Museum (Feller 1976; Feller et al. 1985). If these coatings had been tested for insoluble matter after eight years, the conservator might have concluded that they had no tendency to cross-link. Yet, a decade later, there would have been convincing evidence that insoluble matter had begun to form. Basic scientific studies and principles support these observations; the theory
The chalking of paints—i.e., the degradation of the vehicle so that the paint begins to flake from the surface—has also been observed to exhibit induction-time behavior (Feller 1974). A situation that resembles this behavior is also encountered in the corrosion of metals that have been protected by a paint or varnish. Graphs of arbitrary “corrosion ratings” often show a period of time in which no noticeable corrosion has occurred. The explanation seems plain enough: corrosion of the metal substrate tends not to occur until the organic coating has begun to fail. This situation need not fit a rigorous definition of induction-time chemistry. Yet from the practical point of view of persons responsible for the care of metal artifacts, there is a time when all goes well. Thereafter, a moment occurs at some point in time, often difficult to predict or to “catch,” at which corrosion becomes evident. When these concepts were initially being reviewed in 1972–77, it was possible to suggest that “most of the measures that are taken, or that should be taken, to conserve organic material are concerned with processes that occur during the induction time of oxidative deterioration and are measures essentially concerned with prolongation of the period of induction” (Feller 1974, 146). This point of view is well understood by polymer chemists. Most recently, Edge et al. (1992, 148) stated again, “It is obvious that the small physico-chemical changes which occur during the early stages of aging, i.e., during the induction period prior to the onset of degradation, will be of prime importance with respect to subsequent stability.” Although specific instances can be documented, the phenomenon of apparent induction time is not universally encountered in deterioration problems. When it does occur, however, it is of fundamental significance in preventive care. |