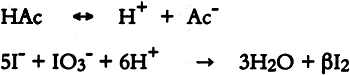TWO TESTS FOR THE DETECTION OF VOLATILE ORGANIC ACIDS AND FORMALDEHYDE
JINPING ZHANG, DAVID THICKETT, & LORNA GREEN
2 THE IODIDE-IODATE TEST FOR VOLATILE ORGANIC ACIDS
2.1 THE REACTION
The iodide-iodate test is based on the reaction of an acid with iodide and iodate ions to produce iodine, which reacts with starch to produce a blue-violet color (Feigl 1954).
Fig. .
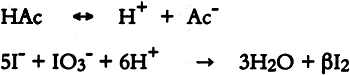 |
Volatile organic acids can be given off by a display material at ambient temperatures. The iodide-iodate test is carried out at elevated temperatures to increase the emission rate of organic acids from the display material under test.
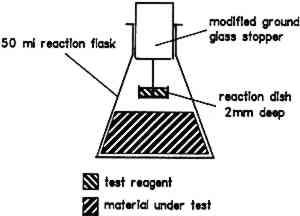
The iodide, iodate, and starch solutions are placed in the reaction dish (fig. 1). The material under test is added to the reaction flask. The stoppered flask is then placed in an oven at 60�C. If the solution in the reaction dish turns blue, this result is a positive indication of volatile organic acids.
Fig. 1.
Spot test apparatus
 |
2.2 FACTORS AFFECTING THE IODIDE-IODATE TEST
- The dimensions of the reaction dish were found to be important. The formation of the blue starch-iodine complex relies on diffusion of the volatile organic acid into the reaction solution. This diffusion is inhibited if the reaction solution is in a deep dish. Trials with dishes of 2 mm and 9 mm depth and with the reaction solution in a small test tube 25 mm deep showed the optimum depth to be 2 mm.
- The temperature at which the test is undertaken will affect the rate of appearance of the blue color. Trials at 60�C, 80�C, and 100�C showed that 60�C is the best temperature at which to conduct the iodide-iodate test. It was found that if temperatures exceeding 80�C were used, no blue color was formed. The high rate of evaporation of the test solution could have inhibited the diffusion of the volatile organic acids into the remaining reaction solution.
- The weight of the sample under test will influence the time taken for the test solution to turn blue. All types of wood will emit varying amounts of volatile organic acids (Arni et al. 1965). The authors carried out two independent tests using different species and weights of wood. The first of these tests involved ash, as sawdust, in weights ranging from 0.025 g to 0.300 g. The time at which the blue starch-iodine complex was first seen was noted. Results are shown in table 1.These results suggested that 0.300 g was not a sufficient sample size for the test. The second test used beechwood cut into blocks (approx. 5 � 5 mm), with weights ranging between 0.045 g and 2.8 g. The time at which the blue complex appeared was again noted. Results are given in table 2.From these tests, a sample weight of 2.0 g was considered most suitable. Reproducible results were obtained from the use of a 2.0 g sample.
- The test solutions have a limited shelf life. The potassium iodide solution was found to deteriorate so that if a solution more than 2 weeks old was used, anomalous positive results were obtained. The potassium iodate and soluble starch solutions were found to be stable for 8 weeks. The soluble starch solution was found to gel within this time and needed warming for redissolution.
TABLE 1 EFFECT OF ASH WOOD WEIGHT ON FORMATION TIME OF BLUE COMPLEX
TABLE 2 EFFECT OF BEECH WOOD WEIGHT ON FORMATION TIME OF BLUE COMPLEX
2.3 RECOMMENDED PROCEDURE
- Prepare the following solutions using distilled water:potassium iodide, KI, 2% (w/v);potassium iodate, KIO3, 4% (w/v); andsoluble starch, 0.1% (w/v).Put two drops of each of the solutions (a)–(c) into the reaction dish shown in figure 1.Add 2.0 g of the sample under test to the reaction flask.Place the stoppered flask in an oven at 60�C.Examine the flask after 30 minutes. If the solution has turned blue, the sample is a source of volatile organic acids and should not be recommended for use with materials known to be susceptible to deterioration in the presence of these acids.
|