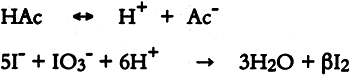TWO TESTS FOR THE DETECTION OF VOLATILE ORGANIC ACIDS AND FORMALDEHYDEJINPING ZHANG, DAVID THICKETT, & LORNA GREEN
ABSTRACT—The iodide-iodate test for the detection of volatile organic acids and the chromotropic acid test for the detection of formaldehyde have been evaluated for use in the selection of materials for the storage and display of antiquities. At present the “Oddy test” with lead is used to identify those materials that have the potential to corrode lead. Since the volatile organic acids and formaldehyde that corrode lead can also affect other materials, the test is also used as an indicator of the evolution of these gases. A comparison with the Oddy test with lead has shown that the chromotropic acid test is more sensitive than the Oddy test and may detect formaldehyde at a level that is unlikely to cause corrosion. The iodide-iodate test and chromotropic acid test take approximately 2 hours to perform compared with 28 days for the Oddy test. The combined use of the iodide-iodate test and the chromotropic acid test has been shown to be a good indicator of the suitability of a material for use in the storage and display of antiquities that are susceptible to volatile organic acids and formaldehyde. However, as the Oddy test is a broader indicator of corrosive gases, its use is recommended when time permits. 1 INTRODUCTIONThere are many reported instances of display materials emitting substances that can damage cultural objects in museums (Oddy 1975; Blackshaw and Daniels 1978; Arni et al. 1965; Brimblecombe 1990). At the British Museum, the “Oddy test” (Oddy 1973) is used to evaluate the suitability of materials proposed for use in the storage and display of antiquities. A disadvantage of this test is that it takes 28 days to complete. Several more rapid tests have been introduced. Daniels and Ward (1982) reported a rapid test using sodium azide for the detection of reducible sulfur compounds that will tarnish silver. A flame test, the Beilstein test, has been used in the British Museum for more than 15 years to detect the presence of chlorine-containing materials. Such materials are considered likely to corrode copper. Williams (1986) published details of this test. Volatile organic acids and aldehydes will cause corrosion of lead (Uhlig 1948) and affect other materials (Green and Thickett 1993; Hatchfield and Carpenter 1987). No rapid tests have been reported for the detection of these substances. The Oddy test with lead is used to detect emissions of volatile organic acids and aldehydes from potential storage or display materials. Two qualitative tests that take less than 2 hours to complete have been investigated. The iodide-iodate test (Feigl 1954) is used to detect volatile organic acids, and the chromotropic acid test (West and Sen 1956) is used to detect free formaldehyde. 2 THE IODIDE-IODATE TEST FOR VOLATILE ORGANIC ACIDS2.1 THE REACTIONThe iodide-iodate test is based on the reaction of an acid with iodide and iodate ions to produce iodine, which reacts with starch to produce a blue-violet color (Feigl 1954).
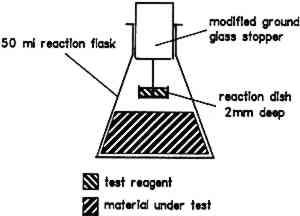
Volatile organic acids can be given off by a display material at ambient temperatures. The The iodide, iodate, and starch solutions are placed in the reaction dish (fig. 1). The material under test is added to the reaction flask. The stoppered flask is then placed in an oven at 60�C. If the solution in the reaction dish turns blue, this result is a positive indication of volatile organic acids.
2.2 FACTORS AFFECTING THE IODIDE-IODATE TEST
TABLE 1 EFFECT OF ASH WOOD WEIGHT ON FORMATION TIME OF BLUE COMPLEX TABLE 2 EFFECT OF BEECH WOOD WEIGHT ON FORMATION TIME OF BLUE COMPLEX 2.3 RECOMMENDED PROCEDURE
3 THE CHROMOTROPIC ACID TEST FOR FORMALDEHYDE3.1 THE REACTIONThe chromotropic acid test is based on the reaction of formaldehyde with a solution of chromotropic acid (1,8-dihydroxynaphthalene-3,6-disulfonic acid) to produce a purple species in solution (West and Sen 1956). The mechanism of this reaction has not been fully elucidated. The test is carried out at elevated temperatures to increase the emission rate of formaldehyde from the display material under test. The spot test presented here has been adapted from the original quantitative method. The chromotropic acid solution is placed in the reaction dish (fig. 1). The material under test is added into the reaction flask. The stoppered flask is then placed in an oven at 60�C until the solution in the reaction dish becomes purple. 3.2 FACTORS AFFECTING THE CHROMOTROPIC ACID TEST
The optimum sample size was found to be 2.0 g TABLE 3 EFFECT OF PLYWOOD WEIGHT ON FORMATION TIME OF PURPLE SPECIES 3.3 RECOMMENDED PROCEDURE
4 COMPARISON OF THE IODIDE-IODATE AND CHROMOTROPIC ACID TESTS WITH THE ODDY TEST4.1 THE ODDY TESTThe Oddy test is used routinely in many museums, including the British Museum (Oddy 1973). In this test, a sample of material is enclosed with a coupon of cleaned metal. Corrosion is accelerated by adding water to create high humidity and by elevating temperatures (60�C). After 28 days, the extent of corrosion on the metal coupon is used to evaluate the suitability of the material under test for use in the display or storage of artifacts containing that metal. A range of materials commonly used for storage or display of antiquities was tested using the iodide-iodate test, the chromotropic acid test, and the Oddy test with lead. Lead is highly susceptible to the corrosive effects of both volatile organic acids and formaldehyde. Four of these materials were chosen to be tested for free formaldehyde using a quantitative aqueous extraction method, modified BS6806, part 2 (British Standards Institution 1987). This method also uses chromotropic acid, but the intensity of the color of the solution is measured using an ultraviolet/visible spectrophotometer. 4.2 DISCUSSIONThere were no instances in which the results of both the chromotropic acid and iodide-iodate tests failed to identify a material that had caused corrosion of lead in the Oddy test and hence was classified as unsuitable for use with lead. In two instances (BM reference numbers 2065 and 2532), the evolution of formaldehyde was detected by the chromotropic acid test, but no corrosion was seen on the lead from the Oddy test. Using the aqueous extraction method, the material BM reference number 2065 contained 336 mg/kg of free formaldehyde. This material produced a purple color in the chromotropic acid test (i.e., gave a positive test result), but it did not cause corrosion of lead in the Oddy test. On testing the material BM reference number 2517, 1177 mg/kg of free formaldehyde were detected. This material did cause the lead coupon in the Oddy test to corrode. These results suggest that the chromotropic acid test will detect levels of formaldehyde that are too low to cause corrosion. 5 CONCLUSIONSResults from the iodide-iodate and chromotropic acid tests correlated well with those from the Oddy test with lead for the particular materials tested. The chromotropic acid test is The iodide-iodate test is specific to volatile organic acids, and the chromotropic acid test is specific to formaldehyde. The major causes of lead corrosion in museums are considered to be volatile organic acids and formaldehydes. However, other species—such as phenol or ammonia, which can be present in some adhesives—have been reported as being corrosive to lead (Evans 1937; Oddy 1975). These species will not be detected by the iodide-iodate test or chromotropic acid test, but they would cause corrosion of lead in the Oddy test. TABLE 4 RESULTS OF COMPARATIVE TESTS WITH A RANGE OF MATERIALS Although the two tests have an advantage over the Oddy test because they take less than 2 hours to complete, they do not replace the Oddy test with lead, which detects the presence of any vapor corrosive toward lead. 6 HEALTH AND SAFETY DATAChromotropic acid: Irritant to eyes and respiratory system. Use in a fume hood, and wear gloves and goggles. Sulfuric acid (concentrated): Corrosive. Wear gloves and goggles when handling. ACKNOWLEDGEMENTSThe authors would like to thank Vincent Daniels and Susan Bradley of the Conservation Research Section, British Museum, and Dekang Zhao of the Conservation Laboratory, Museum of Chinese Revolution, for advice on the investigation and comments on the manuscript. They would also like to thank Andrew Oddy, keeper of the Department of Conservation, REFERENCESArni, P. C., G. C.Cochrane, and J. D.Gray.1965. The emission of corrosive vapours by wood. Part 1. Survey of the acid-release properties of certain freshly felled hardwoods and softwoods. Journal of Applied Chemistry (London) 15: 305–13. Blackshaw, S. M., and V. D.Daniels.1978. Selecting safe materials for use in display and storage of antiquities. ICOM Committee for Conservation preprints, Fifth Triennial Meeting, Zagreb, 23/2/1–9. Brimblecombe, P.1990. The composition of museum atmospheres. Atmospheric Environment24B: 1–8. British Standards Institution. 1987. Formaldehyde in textiles: Method for determination of “free” formaldehyde. BS6806, part 2. British Standards Institution. Daniels, V. D., and S.Ward.1982. A rapid test for the detection of substances which will tarnish silver. Studies in Conservation27: 58–60. Evans, U. R.1937. Metallic corrosion, passivity, and protection. London: E. Arnold and Co. 387. Feigl, F.1954. Spot tests 2: Organic applications. Amsterdam: Elsevier. 95–96. Green, L. R., and D.Thickett.1993. Modern metals in museum collections. In Saving the twentieth century: The conservation of modern materials, ed.D.Grattan.Ottawa: Canadian Conservation Institute. 261–89. Hatchfield, P., and J.Carpenter.1987. Formaldehyde: How great is the danger to museum collections?Cambridge, Mass: Harvard University Art Museums. Oddy, W. A.1973. An unexpected danger in display. Museums Journal73: 27–28. Oddy, W. A.1975. The corrosion of metals on display. In Conservation in archaeology and the applied arts, ed.N. S.Brommelle and P.Smith.London: International Institute for Conservation. 235–37. Sparkes, A. J.1986. The effects of surface coatings on formaldehyde emission. Preprints of Scottish Society for Conservation and Restoration/Museum of Antiquities Symposium on Environmental Monitoring and Control. 78–87. Uhlig, H.1948. The conservation handbook. London: Chapman and Hall. 219. West, P. W., and B.Sen.1956. Spectrophotometric determination of traces of formaldehyde. Journal of Analytical Chemistry153: 12–18. Williams, R. S.1986. The Beilstein test. Canadian Conservation Institute Notes17:1. SOURCES OF MATERIALSChromotropic acid:Sigma Chemicals Co. Ltd., Fancy Rd., Poole, Dorset BH17 7BR, England All other chemicals, Analar Grade:Merck Ltd., Merck House, Poole, Dorset BH15 1TD, England The modified ground glass stopper with suspendedreaction dish (fig. 1) is no longer manufactured in the United Kingdom and has to be made specially. AUTHOR INFORMATIONJINPING ZHANG received an honors degree in microbiology from Shandong University, China, in 1982. He is technical director of Shishong Decorating Materials, Ltd., and he worked in the Conservation Laboratory of the Museum of Chinese Revolution, Beijing, until 1992. From October 1989 to August 1990 he was an intern in the British Museum Department of Conservation, where he undertook the initial work on the iodide-iodate test under the guidance of Vincent Daniels. After his return to China, he began to develop the chromotropic acid test for use in conservation (at the same time as work on this test was independently in progress at the British Museum). He is currently working on second-generation acrylic adhesives and on nonsolvent resin systems, for which he has filed patent applications. Address: No. 6, Suite 1, No. 8 Gong Yuan West St., Jian Guo Men Nei St., Beijing, 100005, PR China. DAVID THICKETT received an honors degree in natural sciences from Cambridge University in 1988. He carried out research for two years in the ceramics industry and joined the Conservation Research Section of the British Museum Department of Conservation in 1990. He conducts testing of materials for storage and display purposes. His current research includes consolidants for degraded amber and methods of removal of polyethylene glycol wax from stone.Address: Department of Conservation, British Museum, London WC1B 3DG, England. LORNA GREEN received an honors degree in chemistry from London University in 1984. After one year of academic research, she joined the Conservation Research Section of the British Museum Department of Conservation. She has a continuing interest in problems associated with display and storage materials and other environmental concerns. At present, she is conducting research on the storage of iron. Address: Department of Conservation, British Museum, London WC1B 3DG, England.
 Section Index Section Index |