THE HARVARD GLASS FLOWERS: MATERIALS AND TECHNIQUESRIKA SMITH McNALLY, & NANCY BUSCHINI
ABSTRACT—The Center for Conservation and Technical Studies, Harvard University Art Museums, recently carried out a preservation study of the Harvard Glass Flowers for Harvard University's Botanical Museum. The study included technical examination of materials, environmental evaluation, and recommendations for exhibition area improvements and conservation treatment. This paper describes the analysis of the materials used to make the models and summarizes the fabrication techniques. Glass, paint, pigments, and adhesives are identified. 1 INTRODUCTIONThe Ware Collection of Glass Models of Plants in the Botanical Museum at Harvard University was created between 1886 and 1936 by Leopold and Rudolph Blaschka, a father-and-son team who had a workshop near Dresden, Germany. Commissioned for Harvard by Elizabeth Ware and her daughter, Mary Lee Ware, in memory of Charles Eliot Ware, the collection was the idea of George Goodale, the first director of Harvard's Botanical Museum. He thought that glass models would capture the beauty and intricacy of plants far better than the papier-m�ch� or wax models of the time. The models would also form a permanent botanical collection for the university. Leopold and Rudolph Blaschka were well-known glass artisans, having established their reputations as fabricators of glass models of marine invertebrates that were sold to museums around the world (including the Museum of Comparative Zoology at Harvard). Although Leopold Blaschka once had ventured into making botanical models, he had settled into the fabrication of marine invertebrates by the 1880s. Goodale, having seen the invertebrate models, convinced the Blaschkas to use their knowledge of glass to create botanical models. Harvard was the Blaschkas' sole employer for the more than 50 years it took to produce the Ware Collection of Glass Models of Plants. The “Glass Flowers,” as the collection is known, are valued for both their scientific and their artistic qualities. They attract nearly 200,000 visitors each year. The collection consists of approximately 850 full-scale models and more than 3,000 enlarged details of plants from all over the world (fig. 1). Visitors are often amazed at the lifelike qualities of the models, and of course many have wondered how they were made. The museum published a small leaflet in 1961, drawing primarily on letters written by Mary Lee Ware at the time the models were made (Ware 1961), and a catalog in 1982 to help explain the materials and techniques of the collection (Shultes and Davis 1982). The models had not been examined in detail by conservators or analyzed by scientific means until this study, which compared written documentation from the university's archives with the results of examination and analysis to determine the materials and techniques.
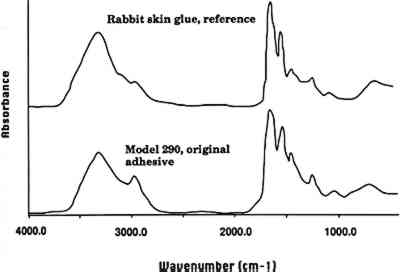
What surprises many people is that the Glass Flowers are not made simply of glass. Many are painted (particularly models made 2 GLASS ANALYSIS: ELECTRON BEAM MICROPROBETo identify the type of glass used to make the Glass Flowers, the Botanical Museum provided 30 fragments collected from damaged models. Of these, 14 were chosen for analysis using electron beam microprobe. In selecting the fragments to be analyzed, an effort was made to obtain an even distribution of fabrication dates within the 50-year working period in order to detect any change or evolution in materials. In addition, since the majority of available fragments were leaf parts, these fragments were selected when possible to provide a foundation for the comparison of glass type and colorants over time. The presence and amounts of the oxides of 13 elements were sought in each sample (table 1). TABLE 1. THE WARE COLLECTION OF BLASCHKA GLASS MODELS OF PLANTS ELECTRON BEAM MICROPROBE ANALYSIS OF GLASS COMPOSITION (WEIGHT PERCENTAGES) 2.1 GLASS, ENAMEL, AND COLORANTSThe analyses indicate that the glass used by the Blaschkas was a soda glass containing a smaller amount of potassium as the secondary alkali. It appears that the Blaschkas used a similar type of glass over the 50-year working period, as the elemental composition of the analyzed samples is fairly consistent. After Leopold's death in 1895, The microprobe analysis indicates that a much wider range in the elemental composition of the enamel was used to color the surface of the clear glass substrate in the years 1895–1936, probably reflecting Rudolph Blaschka's keen interest in enamel and his experimentation with it. After Rudolph first began using colored glass and enamels in 1895, he wrote to Mary Lee Ware of the difficulties he was encountering with the new technique for coloring the botanical models: “The working with the colored glass is specially more exposed to risk in springing and some other unpleasant properties … so it is requisite to work in awfully hot temperatures, on purpose to prevent this damage” (Blaschka 1896). From the letters in the archives, it appears that the biggest problem was springing and shattering of the glass as it was enameled or after enameling as it was annealed and cooled. Annealing is the process of placing a completed glass object back into a section of the glass furnace or oven to allow it to heat uniformly and then cool gradually, releasing strains that have built up in the glass during the forming process and preventing sudden fracture. When Leopold and Rudolph worked together, the technique involved only lampworking (see sec. 5) without the additional complication of enameling over the clear glass. Rudolph also had problems with color in the enamel, including color changes upon reheating. Letters show that he purchased a new hearth and a muffle stove for enameling and annealing as he experimented with the new techniques. Enamel is made of finely ground glass mixed with a medium such as oil or gum, applied to the surface of a clear glass substrate and then heated to melt the powdered glass and fix it to the substrate, in the process burning off the medium. Although the term “enameling” is often used to describe the application of glass to metal, it is also used to refer to glass on glass and was referred to in this way by Rudolph Blaschka. Seven samples of enamel from the Glass Flowers were analyzed using the microprobe, and all contained lead oxide, ranging widely from 8% to 61%, with corresponding decreases in alkali and silica and an overall decrease in lime. The lead glass has a lower melting point than the alkaline glass, allowing Rudolph to apply and then heat the enamel without melting the clear glass substrate. The wide range of composition may be the result of Rudolph's experimentation with melting temperatures and/or the effect of lead on color. Rudolph appears to have searched continually for better heating and melting properties and better color, for as late as 1906 he wrote of the difficulty he was encountering getting the right color for the model of autumn maple leaves. The microprobe analysis indicates that metal oxide colorants were used in the colored glass and enamels from the period 1895 to 1936 (in the earlier period, 1886–95, only clear glass was used). The deep green glass used for some of the leaves contained an average of 1.4% chromium oxide and TABLE 2 GLASS AND ENAMEL COLORANTS (WEIGHT PERCENT) 3 ADHESIVES AND COATINGS3.1 THE BLASCHKAS' ORIGINAL ADHESIVELetters from the Blaschkas to the Wares and to Goodale at the time the models were made suggest that an unnamed glue was used to adhere some parts of the models together. Visual examination and solvent tests of the adhesive used on three models (Phyllocactus ackermanni, Cestrum elegans, and Datura) indicate that this adhesive was hide glue. Fourier transform infrared (FTIR) microspectrometry (Baker et al. 1988) on samples removed from these three models indicated that the adhesive is proteinaceous. This result combined with the results of solvent tests led us to conclude that the adhesive is animal (or hide) glue (fig. 2, spectra 1). Since only three samples were available for analysis of the adhesive, it is certainly possible that Blaschkas also used other adhesives. However, visual examination indicates that the adhesive used in many of the models is probably an animal glue.
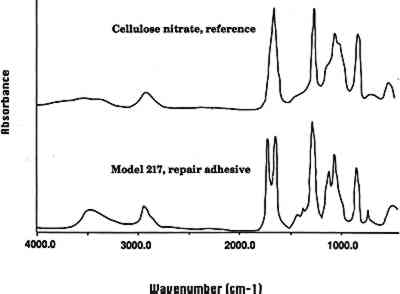
3.2 REPAIR ADHESIVEIn a general condition survey of the collection, a number of previous repairs were noted. Visual examination indicated that the repairs may have been made with a cellulose nitrate adhesive. Some of the repairs are clumsy, and excess adhesive, yellowed in color and containing air bubbles, is visible. The adhesive fluoresces yellow in ultraviolet light and is soluble in acetone. FTIR analysis of repair adhesive from the model C. elegans (model 217, 1891) confirmed that the adhesive is a cellulose nitrate (fig. 2, spectra 2). Given the dates when the models were made (1886–1936), it is possible that in some instances a cellulose nitrate adhesive was also used as an original adhesive. However, visual examination indicates that the original adhesive in many of the models is hide glue. Occasional repair of the models has continued, most probably using a range of modern adhesives.
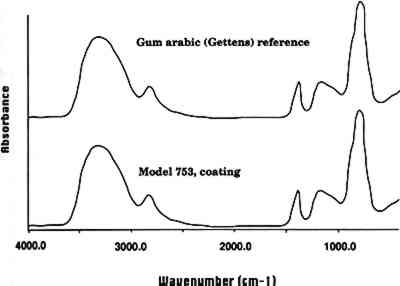
3.3 COATINGSIn 1928, Mary Lee Ware visited Rudolph Blaschka in his workshop near Dresden to see how the models were made. In a letter written to Oakes Ames at the Botanical Museum, she noted that after Rudolph made the glass models and applied color by enameling them, he applied a varnish to the surface (apparently to give the models a more matte appearance, as the glass itself was glossy) (Ware 1928). We examined a leaf of the model Pitosporum tobira (model 753, 1913) and found that it had a matte varnish on the surface that was readily soluble in water. When a small area of coating was removed, the glass underneath was glossy. FTIR analysis indicated that this coating is a carbohydrate. The sample spectrum closely matched that of a reference sample of gum arabic (see fig. 2, spectra 3). Given its ready solubility in water and the FTIR results, this varnish is clearly a plant gum, most probably gum arabic.
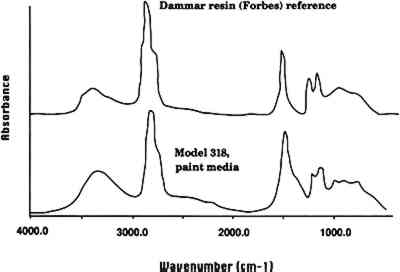
A leaf of the 1929 golden pear was also examined. The coating on the leaf had a faint yellow fluorescence under ultraviolet light and was easily removable with acetone. Like the glass of P. tobira, the glass underneath the coating on this model is glossy. FTIR analysis of a sample of this coating did not give a definitive identification except that it appears to be a natural resin. There were spectral peaks similar to both dammar and copal reference standards, and we conclude that it is a natural resin. The analyses and visual examinations of the models indicate that many indeed have an organic coating applied to the surface, in most instances to make it more matte and give a more lifelike appearance of leaves, stems, and petals. The FTIR analyses of the two coatings agree with Mary Lee Ware's description of the application of varnish. Additional analyses may identify further coating materials. Given that the paint media identified included natural resins and hide glue (see sec. 4.1), it appears that the Blaschkas used a variety of organic media. 4 PAINT AND PIGMENT ANALYSIS4.1 PAINT MEDIALetters from the Blaschkas to Goodale and the Wares at the time the glass models were made indicate that paint was used to color the models, at least in the earlier period of 1886–95. Indeed, in one letter in the museum's archives, Rudolph Blaschka described that he “painted and daubed like a Rembrandt” (Blaschka 1900). The FTIR spectrum of the green paint on P. ackermanni (model 318, 1892) indicated that the binder is probably a natural resin or perhaps a mixture of natural resins. A search of a reference library of spectra of natural resins gave dammar as the closest match (fig. 2, spectra 4). The paint binder on the green painted leaves of the Datura (model 291, 1892) was analyzed and identified as hide glue. Other visual examinations and solvent tests revealed that paint was soluble in either water or acetone. We conclude that the Blaschkas used a variety of organic media to paint the models, possibly even using different media on the same model.
Rudolph Blaschka wrote in a 1906 letter to Mary Lee Ware that the underside of the red maple leaf model (model 726 B) is painted in oil (the rest of the color on this model was achieved by colored glass or enamel) (Blaschka 1906). To date we have not identified any use of oil paint on the models, and the maple leaf model is not 4.2 PIGMENT IDENTIFICATIONPigment samples from 13 of the cold-painted models were analyzed using polarized light microscopy and x-ray fluorescence spectroscopy in a scanning electron microscope. The samples included a range of hues: yellow, red, pink, white, orange, blue, and green (six samples were analyzed). The results indicate that the colors were composed of mixtures of pigments. These are summarized in table 3. TABLE 3 ANALYSIS OF PIGMENT SAMPLES The organic dyes present in the red, pink, and blue samples consisted mainly of a red colorant. The use of dyes is of concern, as the Botanical Museum had wondered if some of the colors had faded. Fading is not apparent, except possibly on a few purple flowers. Although we did not specifically identify the red dyestuff (or dyestuffs), red dyes that would have been available at the time the models were made are susceptible to fading. Ultraviolet light filters and lowered light levels have been recommended to prevent further alteration of the colors containing dyes. 5 SUMMARY OF MATERIALS AND FABRICATION TECHNIQUESThe Glass Flowers are made of a binary alkali glass. The models were made by the glass technique known as “lampworking,” in which the glass is heated in a flame until it becomes soft and malleable and then pulled and twisted into shape with pincers, tweezers, and other tools. Between 1886 and 1895, the color was achieved by cold-painting the cooled surface of the glass with paint made with a variety of media (dammar, hide glue, and gum arabic were identified), using pigments readily available at the time. Beginning in 1895, Rudolph Blaschka experimented with colored glass and with enameling on top of the clear glass substrate. He noted many technical difficulties with this process, including alterations in color from reheating and an inability to anneal the glass shapes properly after enameling. Analysis indicated that the enamel is made of lead glass, which would melt at a lower temperature than the soda glass substrate. The amount of lead oxide in the composition of the enamel varied widely, suggesting Rudolph's experimentation. In this period, varnishes were used to make the glossy surface of the enameled glass more matte. Occasionally, Rudolph also painted the enameled models if he was not satisfied with the color. The Blaschkas employed a wide variety of fabrication techniques, relying on their understanding of glass combined with their The delicacy and ingenuity of Leopold and Rudolph Blaschka's work is more apparent the more one studies the models. The range of fabrication techniques they used reflects the variety of flora they imitated, from small wildflowers to orchids to banana leaves. ACKNOWLEDGEMENTSACKNOWLEDGMENTS The authors would like to thank Henry Lie, director of the Center for Conservation and Technical Studies, Harvard University Art Museums, for his support throughout the project. We thank Eugene Farrell, senior conservation scientist, and Amy Snodgrass, assistant conservation scientist, for their assistance with the analysis. We would also like to thank Susan Rossi-Wilcox of the Botanical Museum for instigating the study. REFERENCESBaker, M., D.von Endt, W.Hopwood, and D.Erhardt. 1988. FTIR microspectrometry: A powerful conservation analysis tool. AIC preprints, 16th Annual Meeting, American Institute for Conservation, Washington, D.C. 1–13. Blaschka, R.1896. Letter to Mary Lee Ware, December 1, 1896. Botanical Museum Archives, Harvard University. Blaschka, R.1900. Letter to Mary Lee Ware, August 27, 1900. Botanical Museum Archives, Harvard University. Blaschka, R.1906. Letter to Mary Lee Ware, October 28, 1906. Botanical Museum Archives, Harvard University. Newton, R., and S.Davison. 1989. Conservation of glass. London: Butterworth. Shultes, R. E., and W. A.Davis. 1982. The glass flowers at Harvard. New York: E. P. Dutton. Ware, M. L.1928. Letter to Professor Oakes Ames, October 3, 1928. Botanical Museum Archives, Harvard University. Ware, M. L.1961. How were the glass flowers made?: A letter by Mary Lee Ware. Botanical Museum Leaflets (Harvard University) 19(6) (January 9): 125–36. AUTHOR INFORMATIONEXPERIMENTAL: The cameca MBX electron beam microprobe at the Department of Earth and Planetary Science, Harvard University, has three wavelength-dispersive spectrometers. The system is equipped with a Tracor Northern TN-5502 EDS system and a TN-1310 WDS and stage automation system. The system was calibrated using mineral standards, and a Corning TVX 321 glass standard was run to confirm accuracy. The Bence-Albee matrix correction program was used. RIKA SMITH MCNALLY has a B.F.A. from the Rhode Island School of Design and an M.S. in conservation from the University of Delaware/Winterthur Art Conservation Program. She is associate conservator of objects and sculpture at the Center for Conservation and Technical Studies, Harvard University Art Museums. Address: Center for Conservation and Technical Studies, Harvard University Art Museums, 32 Quincy St., Cambridge, Mass. 02138. NANCY BUSCHINI has a B.S. in biology and art from the University of Massachusetts at Boston and an M.A. in conservation from the Buffalo State College Art Conservation Program. She is assistant conservator of objects and sculpture at the Center for Conservation and Technical Studies, Harvard University Art Museums. Address: Center for Conservation and Technical Studies, Harvard University Art Museums, 32 Quincy St., Cambridge, Mass. 02138.
 Section Index Section Index |