CONSOLIDATION OF POROUS PAINT IN A VAPOR-SATURATED ATMOSPHEREERIC F. HANSEN, ROSA LOWINGER, & EILEEN SADOFF
4 METHODS FOR REDUCING CHANGES IN APPEARANCECurrent methods for minimizing the change in appearance in the consolidation of a high PVC paint are: (1) the use of a low volatility solvent, like diethylbenzene (DEB) (Welsh 1980); (2) multiple applications of a dilute solution; (3) the use of gelatin or cellulose ethers as consolidants (Hansen, Sadoff and Lowinger 1990); and (4) the use of small particle size dispersions of acrylics (Koob 1981). Both of the first two methods appear to be dependent on the same principle introduced above: control of penetration and distribution. Viscosity is the resistance to flow, and in a solution of a thermoplastic polymer the viscosity is higher at increased concentrations of resin. By allowing the viscosity of solutions to remain low long enough for the solution to flow into the paint, maximum penetration and distribution are achieved. This process may avoid localized surface concentrations of resin (reducing darkening) and also possibly improve the adhesion to the substrate if a sufficient amount of material penetrates to the interface. Efficient penetration may be accomplished by ensuring that the concentration, and hence the viscosity, does not increase rapidly as a result of solvent evaporation.
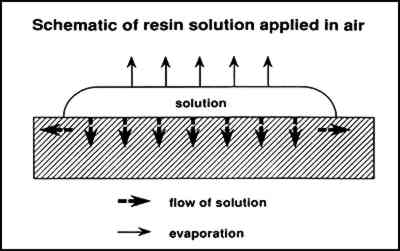
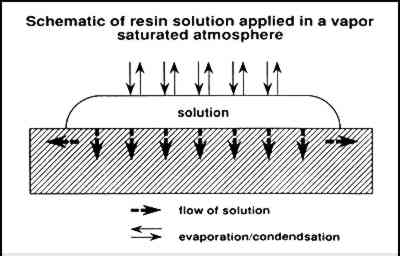
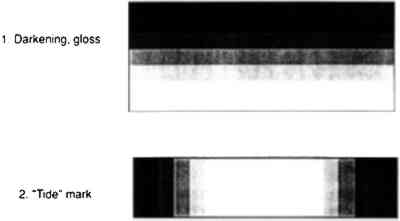
Figure 5 illustrates the flow of a solution into a paint layer shown in cross section while solvent simultaneously is evaporating from the surface. Figure 6 illustrates the condensation and evaporation that occurs in a saturated vapor atmosphere, the net result being negligible loss of solvent. In the first case, the concentration of the solution that initially flows into the paint is lower and therefore at a low viscosity. The concentration increases with solvent evaporation, which may finally result in the concentration profile illustrated in figure 7 (also a cross-sectional view). Greater concentrations are predicted at the surface, resulting in profile 1, causing darkening and gloss. Also occurring simultaneously as the solution flows outward is continual evaporation resulting in greater concentration of resin at the edges away from the center where the solution
In contrast, if solvent loss through evaporation is suppressed, a solution will flow into the paint with little or no increase in resin concentration or viscosity. Resin concentrations will tend to be equal throughout a homogenous paint layer where wetting of pigment particles is sufficient and the paint is not thick enough to produce possible chromatographic effects. When diethylbenzene (a very low volatility solvent) is used, there is little solvent evaporation for an extended time, promoting maximum penetration and spreading. In the case of very dilute solutions, the concentration will increase with solvent evaporation, but the viscosity will remain lower for a longer time in comparison with a solution that is initially at a higher concentration (and is therefore at a higher initial viscosity). Following similar reasoning, we have evaluated another method of minimizing the changes in appearance of matte, powdering surfaces that result from consolidation. This method maintains a low viscosity long enough for maximum penetration and spreading of the solution. An object is treated in an enclosed environment with open pools of solvent that saturate the atmosphere with vapor. After application of a consolidant by brush or spray, the object is allowed to remain until penetration is extensive. Because solvent volatility is no longer a significant factor, very volatile solvents such as acetone may be used successfully. An important point of this method is that the solution is applied in a saturated atmosphere rather than applied to the surface of an object that is then placed in a saturated atmosphere, a common conservation practice to reduce reverse migration. |