CONSOLIDATION OF POROUS PAINT IN A VAPOR-SATURATED ATMOSPHEREERIC F. HANSEN, ROSA LOWINGER, & EILEEN SADOFF
ABSTRACT—When resins are applied for consolidation of a matte paint surface, the colors may darken because of increased surface gloss, the result of the formation of a film over the surface to be protected. Darkening may also occur when resin fills the void space in the powdering paint. A technique is described for consolidating matte, low-gloss, powdery surfaces with minimal if not negligible darkening of the colors. The technique outlined involves the application of a stable thermoplastic resin solution in an atmosphere containing a very high concentration of the solvent used to dissolve the resin, thus slowing solvent evaporation and allowing the resin to penetrate the paint layer and surround the exposed pigment particles. In addition to retaining the matte appearance of the object, “tide lines” may be prevented and adhesion of the pigment may be improved. 1 INTRODUCTIONThis article presents what we hope will be a useful technique—the application of a consolidant solution in an atmosphere having a high concentration of the same solvent used in formulating the solution—along with some simple visual evidence that such a procedure is more effective than certain procedures used in the past. The information is designed to be of direct benefit to the practicing conservator; some practical considerations are noted based on the authors' experience. The factors affecting the appearance of matte paint are briefly discussed, and a physical explanation of the processes by which this technique minimizes changes in appearance is presented. Paints that have a high pigment volume concentration (PVC) may be light and matte in appearance. In addition, the paint may be friable or powdering due to the lack of resin or binder. Consolidation may be required to prevent loss of paint from the surface. However, high PVC paints are also porous, and they easily absorb consolidant solutions, with the result that darkening or discoloration can occur. Other undesirable results of a consolidation treatment may include increased gloss and the formation of “tide lines” (see sec. 3). Coatings with a high PVC are often encountered in at least three widely different classes of objects. Ethnographic objects may have been painted using such pigments as kaolin and ochres applied from a water slurry, containing no binder at all. Small amounts of binders may have been added, or organic material of limited durability may have been used. Modern works of art may have a high PVC paint if an artist specifically tried to achieve a matte appearance or experimented in novel paint formulations. Medieval panel paintings executed with water-soluble binders, glue, or resins may be The results of consolidating powdering, matte paint under different conditions are compared on the basis of a qualitative visual rating scale (sec. 6.2). Wooden blocks painted with yellow ochre, red ochre, and kaolin, prepared with no binder, were consolidated with the acrylic resin Acryloid B-72 or the poly(vinyl acetate) resin AYAF in solutions made with solvents of differing volatilities (Table 1). The consolidant solutions were applied in two ways: in air and in an atmosphere saturated with the solvent used to dissolve the resin. The results of these tests and the implications of this method for the consolidation of a wide variety of objects with powdering surfaces are discussed, along with suggestions for optimizing the procedure.
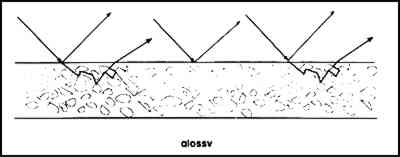
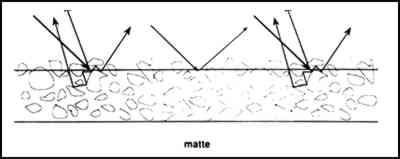
2 MATTE PAINT: SURFACE ROUGHNESS AND PIGMENT VOLUME CONCENTRATIONThe paint industry describes the concentration of vehicle and pigment in a coating based on the volume percent instead of the weight percent, because many of the properties of coatings vary according to the ratio of the pigment volume to the vehicle volume, not with the ratio of the pigment mass to the vehicle mass. An important consideration is the critical pigment volume concentration (CPVC). Above the CPVC void spaces are present. Below the CPVC the pigment particles are entirely surrounded by binder. Abrupt changes in many paint properties tend to occur at the CPVC (Patton 1979). In addition to becoming lighter in color with increasing void space, tensile properties decrease and porosity increases as the pigment volume concentration increases above the CPVC. The general reasons why paint becomes lighter as the pigment volume concentration rises above the CPVC are illustrated in figures 1–3. A film below the CPVC has an excess of resin and may exhibit a smooth surface that specularly reflects light (fig. 1). This light is perceived as gloss. Above the CPVC there is less vehicle present, and, if
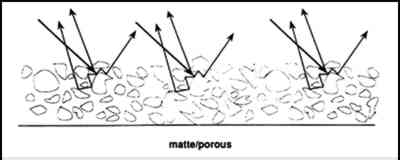
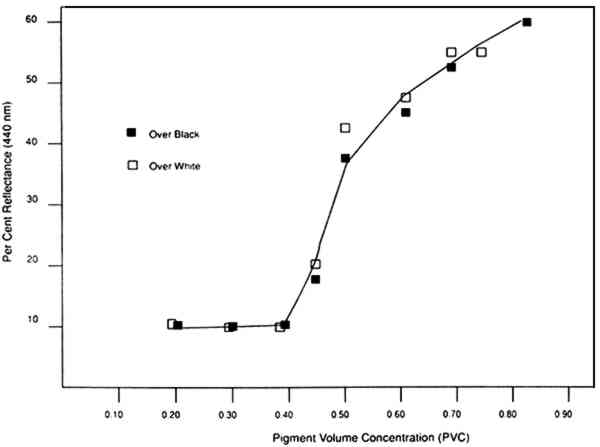
When the volume of pigment is increased sufficiently, scattering also increases due to the greater volume of air/pigment interfaces (fig. 3). This increased amount of scattered, or diffuse, light is further additively mixed as white. Feller and Kunz (1981) documented the increase in surface reflectance at 440 nm for ultramarine blue paint formulated at various PVCs (fig. 4). This is the wavelength at which the minimum reflectance will occur when the paint is saturated with vehicle or consolidant. Simply put, with increased void space (and therefore greater scattering at the air/pigment interface) the amount of reflected, diffusely scattered light increases, resulting in a light color. To avoid darkening of porous, matte paint by the addition of a consolidant, filling of void spaces and smoothing of the surface must be minimized.
3 CAUSES OF DISCOLORATIONTwo of the many possible explanations for the discoloration or darkening of porous or powdering paint resulting from consolidation are: (1) the differences in the refractive indices of pigment and vehicle; and (2) reverse migration of polymer from the interior to the surface with solvent evaporation. These effects are considered to be of little significance in this study.
Feller and Kunz (1981) initiated their study in part to demonstrate that the darkening phenomenon resulted primarily from the filling of void spaces. A series of polymers of different refractive indices were shown to be similar in their darkening effects for paint formulated with ultramarine blue at different A high surface concentration of consolidant has been explained as a result of the migration of a resin solution that has penetrated a porous object back to the surface of the object with solvent evaporation as a consequence of capillary action (Wilks 1987). This process is often termed “reverse migration.” Reverse migration has been extensively discussed in relation to stone consolidation by Domaslowski (1987–88). He considers the migration process to be insufficiently known and understood by both conservators and scientists. He states that the resin migration effect depends on the quality of solvents, dimensions of resin molecules, solution viscosity, stone structure, and drying conditions after impregnation. He found that the most important factor in limiting reverse migration is the solvent “quality,” or the tendency of the dissolved polymer to remain dissolved in a solvent. Poor solvents for polymers resulted in polymer deposition and lack of migration, provided that the polymer sufficiently penetrated the stone pores. (In some instances the solution viscosity is too high to allow sufficient penetration into the stone, leaving a higher concentration of resin at the surface.) Domaslowski found that migration did not follow a sequence equivalent to solvent volatility rates, except that migration was reduced for very low volatility solvents. He also found that slow, long-term drying may reduce migration in solvents of weak and moderate quality. To achieve slow drying, resin migration was prevented by placing stone impregnated with solution in an enclosed container with vapors of “white spirits.” It is important to be aware of reverse migration because we are not taking the position in this article that working in a solvent vapor–saturated atmosphere reduces reverse migration and subsequent discoloration resulting from high surface concentrations of resins. Instead, our working assumption is that surface concentrations of resins are avoided when solvent loss through evaporation is inhibited (sec. 4). Johnston-Feller (1990) suggested that surface concentrations of the resin remaining after treatment with a consolidant are due primarily to lack of penetration, lack of efficient wetting of pigment particles, and uneven distribution of resin solution within the paint. It was further suggested (Wicks 1990) that this lack of penetration is the result of an increase in solution viscosity due to solvent evaporation during the time it takes for a solution to penetrate a porous paint and that this effect could be reduced by minimizing or eliminating solvent loss until desired penetration and spreading of the solution were achieved. Schiessl (1989) has discussed certain conditions of a matte paint that may result in discoloration from consolidation with a solution of a resin and that cannot be minimized by inhibiting solvent loss during application. He has commented on the formation of “tide lines,” observing that this phenomenon sometimes results either from of the solubilization of dirt, fungus, and bacteria or from the physical redistribution of small particles in a paint that has a wide range of pigment particle size. As can be observed through a microscope, application of a solution moves the smaller particles, leaving the larger particles in place. 4 METHODS FOR REDUCING CHANGES IN APPEARANCECurrent methods for minimizing the change in appearance in the consolidation of a high PVC paint are: (1) the use of a low volatility solvent, like diethylbenzene (DEB) (Welsh 1980); (2) multiple applications of a dilute solution; (3) the use of gelatin or cellulose ethers as consolidants (Hansen, Sadoff and Lowinger 1990); and (4) the use of small particle size dispersions of acrylics (Koob 1981). Both of the first two methods appear to be dependent on the same principle introduced above: control of penetration and distribution. Viscosity is the resistance to flow, and in a solution of a thermoplastic polymer the viscosity is higher at increased concentrations of resin. By allowing the viscosity of solutions to remain low long enough for the solution to flow into the paint, maximum penetration and distribution are achieved. This process may avoid localized surface concentrations of resin (reducing darkening) and also possibly improve the adhesion to the substrate if a sufficient amount of material penetrates to the interface. Efficient penetration may be accomplished by ensuring that the concentration, and hence the viscosity, does not increase rapidly as a result of solvent evaporation.
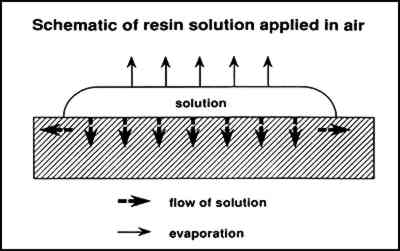
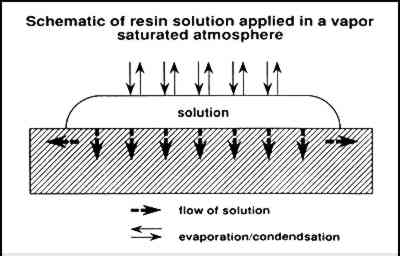
Figure 5 illustrates the flow of a solution into a paint layer shown in cross section while solvent simultaneously is evaporating from the surface. Figure 6 illustrates the condensation and evaporation that occurs in a saturated vapor atmosphere, the net result being negligible loss of solvent. In the first case, the concentration of the solution that initially flows into the paint is lower and therefore at a low viscosity. The concentration increases with solvent evaporation, which may finally result in the concentration profile illustrated in figure 7 (also a cross-sectional view). Greater concentrations are predicted at the surface, resulting in profile 1, causing darkening and gloss. Also occurring simultaneously as the solution flows outward is continual evaporation resulting in greater concentration of resin at the edges away from the center where the solution
In contrast, if solvent loss through evaporation is suppressed, a solution will flow into the paint with little or no increase in resin concentration or viscosity. Resin concentrations will tend to be equal throughout a homogenous paint layer where wetting of pigment particles is sufficient and the paint is not thick enough to produce possible chromatographic effects. When diethylbenzene (a very low volatility solvent) is used, there is little solvent evaporation for an extended time, promoting maximum penetration and spreading. In the case of very dilute solutions, the concentration will increase with solvent evaporation, but the viscosity will remain lower for a longer time in comparison with a solution that is initially at a higher concentration (and is therefore at a higher initial viscosity). Following similar reasoning, we have evaluated another method of minimizing the changes in appearance of matte, powdering surfaces that result from consolidation. This method maintains a low viscosity long enough for maximum penetration and spreading of the solution. An object is treated in an enclosed environment with open pools of solvent that saturate the atmosphere with vapor. After application of a consolidant by brush or spray, the object is allowed to remain until penetration is extensive. Because solvent volatility is no longer a significant factor, very volatile solvents such as acetone may be used successfully. An important point of this method is that the solution is applied in a saturated atmosphere rather than applied to the surface of an object that is then placed in a saturated atmosphere, a common conservation practice to reduce reverse migration. 5 EXPERIMENTAL5.1 MATERIALSWood blocks (kiln-dried boxwood blocks, 3 � 6 � 1 in) were obtained that did not contain resins that would solubilize and discolor the paint. The pigments chosen were ochres and kaolin because the authors were evaluating methods and materials for the consolidation of painted ethnographic wood objects (Hansen, Sadoff, and Lowinger 1990), and these particular pigments are ubiquitous on many such objects. The red ochre and the yellow ochre were supplied in mineral form and ground to a powder in mortar and pestle. The pigments were dispersed by the addition of water alone, without binder, until the paint had a consistency that allowed application by a brush (equal volumes of water and powdered pigment). A kaolin dispersion was first applied to complete hiding of the block. Then a simple geometric pattern was painted on in red and yellow ochre. The layers were allowed to dry completely (more than 72 hours) before application of consolidants. 5.2 CONSOLIDATIONSolution concentrations were 5% (w/v) PVAC AYAF in acetone, ethanol, and toluene and 5% (w/v) Acryloid B72 in acetone, toluene, xylene, and diethylbenzene. Application of the pure solvents to the painted blocks resulted in no discernible change in appearance once the solvents had dried. The solutions were applied to approximately 1-in wide areas of the sides of the blocks in two different environments: (1) open to air and (2) in a commercially available, sealable glove-bag of 3 mil polyethylene (an Atmosbag) saturated with the solvent used to dissolve the resin and placed inside a fume hood. The central area was left unconsolidated to allow visual comparison of treated and untreated areas. For each of the seven solutions, the Atmosbag was set up (fig. 8) to contain the following before sealing:
After the blocks were sealed in the Atmosbag, a period of 30 to 45 minutes passed before the consolidant was applied to the surface. The brush in use was cleaned with the appropriate solvent and the excess removed with paper towel every time the brush was removed from a painted surface and/or before treating a “fresh” area with a consolidation solution. Solutions were swirled as often as necessary to keep the concentration constant. Three methods of application were used:
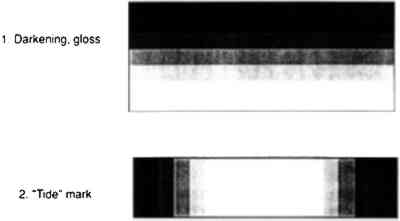
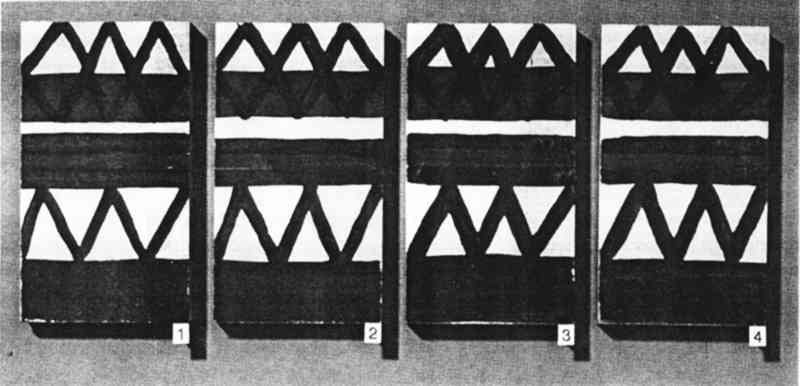
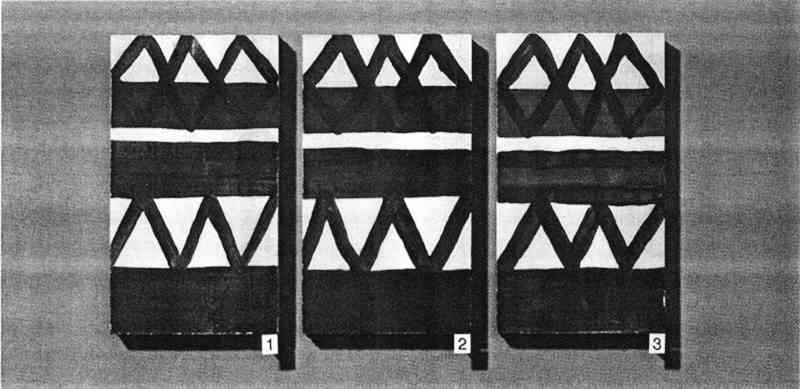
Following treatment in the bag, the blocks were left 5–10 minutes, allowing the solution to penetrate. Penetration could be observed visually, as the area to which the solution was applied slowly became indistinct in comparison to the original observed darkening of the wet area. The treated block was then removed and left to air dry. Blocks were numbered for identification and comparison, and they were photographed before and after treatment. Photography proved useful because the original painted surfaces had some streaking and lines that could be misinterpreted as tide lines due to consolidation. 6 RESULTS AND DISCUSSION6.1 CHOICE OF MATERIALS AND METHODSThe ochres and kaolin used in this study could be applied as colorants from an aqueous dispersion with no binder. This type of paint was chosen because such an easily smudged and powdering surface might be encountered in a museum object requiring consolidation. The simple, multicolored geometric pattern was selected for its similarity to many designs found on ethnographic objects. The three methods used to apply the consolidant are those frequently used by conservators. In particular, dabbing does not drag pigments of one color onto an area pigmented with another color, but it may result in tide lines. Different application procedures were employed, because the conservator's skill in applying a consolidant The Atmosbag is an inexpensive device for maintaining a high concentration of solvent in a convenient working space. It comes in a variety of sizes, has gloves attached, and is easily sealable. It has been previously suggested for use in humidifying objects and working on them in a controlled humidity maintained with saturated salt solutions (Maltby 1987). Any other system for creating an enclosed space that is impermeable to solvent vapors and allows both manipulation of objects and visibility—such as glove boxes or glove bags of other materials and designs—would also be suitable. It is important to note that the manufacturers do not recommend the Atmosbag for safe control of solvent vapors, and it should be used in a fume hood, as should any other system. 6.2 EVALUATION SYSTEM AND RATINGIf consolidation by introducing a resin can be avoided and a nonintrusive conservation method—such as proper storage conditions—can be used instead, such a method is usually preferable. This is often not an option, however, for an object with powdering paint; the surface must be consolidated, even if some discoloration results. But the changes resulting from the necessary consolidation can be minimized, though not necessarily prevented. A qualitative rating system was developed to enable conservators to judge whether their work is within acceptable limits. Consolidation strength was estimated by the difficulty of smudging the surface by pressure applied with a finger. Consolidation was acceptable in every case, an expected finding with solutions of a fairly high working concentration (5% w/v). Consolidation was poorer in a few instances when xylene and DEB had been used as solvents. Because consolidation was tested two weeks after treatment, these results may relate to retained solvent alone, since these solvents have low boiling points. Although the effects on appearance listed below are interesting in regard to the correlation of the degree of discoloration with the volatility of the solvent, the more interesting phenomenon is that consolidation in a saturated atmosphere appears to be largely independent of solvent volatility. This result is readily apparent in the color reproductions (figs. 9–10) of the painted blocks consolidated by application method 2, dabbing.
The system used to rate the appearance of the paint after consolidation was:
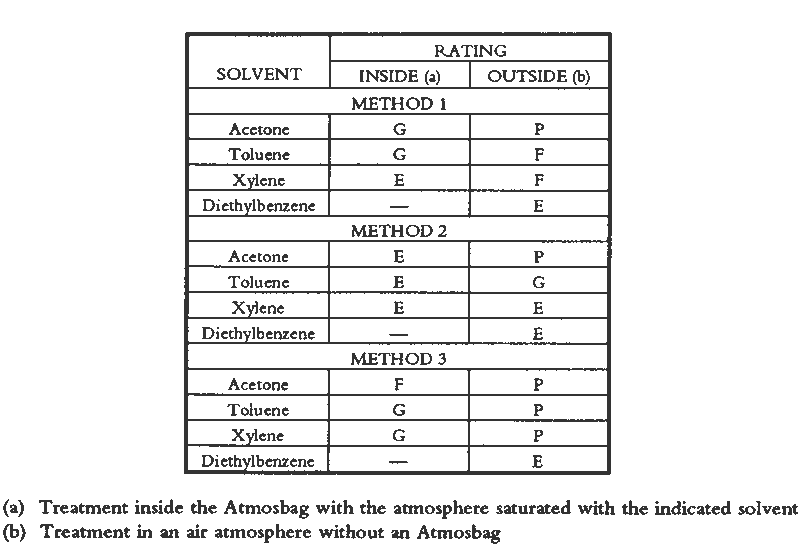
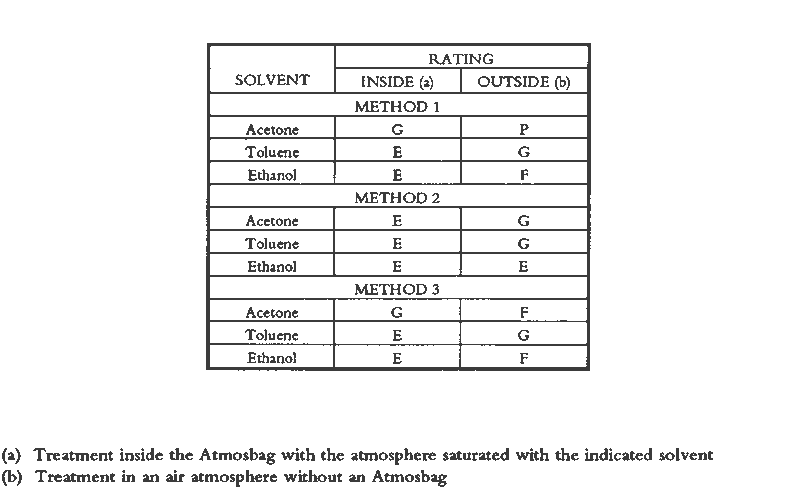
Visual changes two weeks after treatment between consolidated areas and central unconsolidated areas were evaluated without knowledge of the particular solution used. The results (Tables 2, 3) support the following observations. For every solution tested, painted surfaces consolidated inside the bag looked better than those treated outside the bag. The application method is not as important in the case of consolidation inside the bag as it is for outside the bag.
Generally, outside the bag, the higher the volatility of the solvent, the poorer the appearance of the consolidated areas. Overall, outside the bag, the objects treated with AYAF solutions looked better than those treated with Acryloid B72. The one exception was B72/DEB, rated excellent. The best overall appearance outside the bag resulted from using B72/DEB and AYAF/ethanol; the best overall appearance inside the bag resulted when AYAF/ethanol was used; and the worst overall appearance outside the bag resulted from the use of B72/acetone. 7 TOXICITY AND SAFETY FACTORSAs previously mentioned, the AtmosBag's manufacturers do not recommend its use in protection against possibly toxic solvent vapors. It is not always possible to tell if there are small leaks in the system, especially if the bag is being used repeatedly. It is important to work in a fume-hood with a sufficient draft to remove any possible solvent vapors. Taking this precaution does not interfere with the consolidation procedure because the interior of the bag is protected from the air flow. Because of the danger of exposure to high concentrations of organic solvents and the added potential for flammability associated with a vapor-saturated atmosphere, particularly with acetone, general laboratory safety procedures relating to the use of organic solvents should be strictly adhered to. These procedures include sufficient air flow in a fume hood, no open flames, and appropriate electrical systems. 8 PRACTICAL CONSIDERATIONSOne reason for exploring the method of working in a saturated atmosphere is that there are some drawbacks to working with DEB. DEB can be retained in treated objects for a long time, resulting in a noxious odor that may persist for years. In addition, the degree of consolidation or reduction of discoloration is not always acceptable (Walston et al. 1987). The possible reasons for this result include the plasticization of resins when a large amount of solvent is retained and the fact that DEB is not a good-quality solvent for acrylic polymers. Some physical properties of thermoplastic polymers used in conservation have been shown to be affected by the quality of the solvent used for their application (Hansen et al. 1991). There are obvious limitations to the use of a saturated vapor atmosphere, especially in regard to undesirable softening of resinous areas of an object. This problem was apparent quite early in this study when the paint on brush handles softened because it was solvent sensitive. Another consideration is the difficulty in removing a consolidant that is well distributed throughout a fragile paint layer (Hansen, Sadoff, and Lowinger 1990). Only highly light stable, nonyellowing, thermoplastic polymers should be used. Consolidation in a saturated atmosphere is particularly suitable in some situations and not suitable in others. Suitable surfaces are porous ones where penetration can be achieved, either in the painted layer alone or in the paint and substrate. Surfaces that are matte due to surface roughness and not porous, or paint and substrates where improved penetration is not required, should not benefit by this method. Matte surfaces due to surface roughness might be successfully consolidated with viscous solutions that do not penetrate but also do not level well, conforming to the surface geometry. Factors affecting this effect have been discussed by Feller, Stolow, and Jones (1985). In contrast, paint might also be porous and lifting or flaking but not be in need of consolidation. If all that is required is adhesion of the porous paint to the substrate, discouraging penetration into the paint by the adhesive solution would minimize darkening or discoloration. Futernick (1989) found that if a porous paint was saturated with a nonpolar organic solvent, such as toluene, it could be adhered with an aqueous emulsion that did not penetrate into the pores of the saturated paint. 9 CONCLUSIONSA method was devised, working in a saturated atmosphere, that will produce similar minimal changes in appearance as when working with a low-volatility solvent such as DEB. The method was developed and evaluated based on the principle that greater penetration and spreading resulted from solutions of low viscosity when a low-resin concentration was maintained by inhibiting solvent evaporation. The method also differs from placing an object in a saturated atmosphere after consolidation because a broad range of solvent volatilities can be accommodated. By working in a saturated atmosphere, even a highly volatile solvent such as acetone may be used successfully. Simple visual evidence (figs. 9–10) is sufficient to support the conclusion that darkening resulting from consolidation can be minimized by inhibiting solvent evaporation. Further work in this area should involve quantitative studies of appearance change (color measurements), which could provide specific information relating the extent of darkening to concentrations of consolidants, solvent volatilities, solution viscosities, distributions within a paint layer, and adhesive strengths. ACKNOWLEDGEMENTSThe authors would like to thank Robert Feller, Ruth Johnston-Feller, Sue Walston, and Zeno Wicks for comments and suggestions. Portions of this paper were presented at the Objects Specialty Group Session of the AIC Annual Meeting in Richmond, Virginia, 1990. REFERENCESDomaslowski, W.1987–88. The mechanism of polymer migration in porous stones. Weiner Berichte uber Naturwissenschaft in Kunst4/5:402–25. Feller, R. L., and N.Kunz. 1981. The effect of pigment volume concentration paint on the lightness and darkness of porous paints. AIC preprints. Washington, D.C.: American Institute for the Conservation of Historic and Artistic Works. 66–74. Feller, R. L., N.Stolow, and E.Jones. 1985. Picture varnishes and their solvents, Washington, D.C.: National Gallery of Art. 137–45. Futernick, R.1989. Personal communication. Hansen, E. F., M.Derrick, M.Schilling, and R.Garcia. 1991. The effects of solution application on some physical properties of thermoplastic polymers used in conservation: Poly(vinyl acetates). Journal of the American Institute for Conservation30:203–13. Hansen, E. F., E.Sadoff, and R.Lowinger. 1990. A review of the problems encountered in the consolidation of painted ethnographic objects and potential solutions. ICOM Committee for Conservation preprints, 9th Triennial Meeting, Dresden. ed.K.Grimstaad. Los Angeles: ICOM Committee for Conservation. 163–68. Johnston, R. M., and R. L.Feller. 1965. Optics of paint films: Glazes and chalking. In Application of science in examination of works of art. Boston: Museum of Fine Arts. 86–95. Johnston-Feller, R. M.1990. Personal communication. Koob, S. P.1981. Consolidation with acrylic colloidal dispersions. AIC preprints. Washington, D.C.: American Institute for the Conservation of Historic and Artistic Works. 66–74.
Maltby, S.1987. “Atmosbag”: A controlled atmosphere chamber with built-in gloves. Ethnographic Conservation Newsletter3:20–21. Patton, T. C.1979. Paint flow and pigment dispersion. New York: John Wiley and Sons. Schiessl, U.1989. Konservierungstechnische beobachtungen zur festigung wassrig gebundener, kreidender malschichten auf holz. Zeitschrift fur Kunsttechnologie und Koservierung2:293–320. Tess, R. W.1985. Solvents. In Applied Polymer Science, ed.R. W.Tess and G. W.Pohlein. Washington, D.C.: American Chemical Society. 661–700. Welsh, E. C.1980. A consolidation treatment for matte, powdery paint. AIC preprints. Washington, D.C.: American Institute for the Conservation of Historic and Artistic Works. 141–50. Walston, S., D.Horton-James, and S.Zounis. 1987. Investigation into methods and materials for the adhesion of flaking paint of ethnographic objects: A progress report. ICOM Committee for Conservation preprints, 8th Triennial Meeting, Sydney, Australia. K. Grimstaad. Los Angeles: Getty Conservation Institute. 141–50. Wicks, Z.1990. Personal communication. Wilks, H.ed.1987. Science for conservators, book 3: Adhesives and consolidants. London: The Conservation Unit, Museums and Galleries Commission. 127–28. SOURCES OF MATERIALSOchresWard's Natural Science Establishment, Inc., P.O. Box 92912, Rochester, N.Y. 14692–9012 KaolinS. Paul Ward, Inc., 60 Mission St., Box 336, South Pasadena, Calif. 91030 AtmosbagAldrich Chemical Co., Inc., 1001 West Saint Paul Ave., Milwaukee, Wis. 53233 AUTHOR INFORMATIONERIC F. HANSEN is a Fellow of the AIC who graduated from the University of California at Irvine in 1980 with an M.S. in organic chemistry and joined the Getty Conservation Institute in 1985. In his current position as an associate scientist, he is pursuing his interests in the environmentally induced deterioration of synthetic and natural organic materials (including collagen, fibroin, and keratin), the conservation of ethnographic and archaeological objects, and the analysis of the effects of treatment parameters on the final physical and optical properties of treated objects. Address: Getty Conservation Institute, 4503 Glencoe Ave., Marina del Rey, Calif. 90292. ROSA LOWINGER is the director of the Sculpture Conservation Studio, a conservation facility in Los Angeles for the preservation of sculpture and decorative arts. In her 10 years in private practice, Lowinger has worked with numerous institutions and public agencies, including the City of Los Angeles, the National Park Service, and the U. S. Navy. She has an M. A. in conservation from the Institute of Fine Arts, New York University, and has been a fellow at the Los Angeles County Museum of Art and the University Museum, University of Pennsylvania. Address: Sculpture Conservation Studio, 1144 South Stanley Ave., Los Angeles, Calif. 90019. EILEEN SADOFF is employed in the objects conservation laboratory at the Los Angeles County Museum of Art. From 1988–90 she assisted Eric Hansen with the development of the ethnographic training course at the Getty Conservation Institute. She was most specifically involved with facsimile research and manufacture to replicate the problems associated with powdery matte pigments on indigenous artifacts, and with testing of consolidants and application techniques for this type of object. She received a B.A. in art from the University of Minnesota, Address: Los Angeles County Museum of Art, Conservation Center, 5905 Wilshire Blvd., Los Angeles, Calif. 90036.
 Section Index Section Index |