THE RECOVERY AND DRYING OF TEXTILES FROM A DEEP OCEAN HISTORIC SHIPWRECKKATHRYN A. JAKES, & JOHN C. MITCHELL
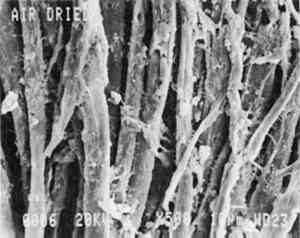
5 RESULTS AND DISCUSSIONThe trunk lining is a coarse, plain-woven fabric with a yarn count of 44 � 51 yarns per in. Examination of trunk lining fibers by light The fabric of the air-dried trunk lining (fig. 1) shows some surface tangling of fibers, but the fabric structure is still apparent. At higher magnification (fig. 2) the fibers appear round, with some eaten-away areas due to microbial damage. The fibers range in size from 10 to 20 μm in diameter. Foamy deposits of contaminants were observed in some areas of the fiber surfaces, but they do not obscure the fabric structure.
Fabric dried from ethanol also displayed some surface tangling. In addition, the fibers appear flattened and stuck together (fig. 3). The fabric structure is not as clear as that of the air-dried sample. Although the samples were taken from the same trunk lining, it is possible that different sections of fabric experienced different stresses during the performance life of the fabric. Yarns that experienced more compressive force will be flattened due to that force. However, if the lack of definition of the fabric structure is due to the drying process, the ethanol dehydration is a much less desirable method.
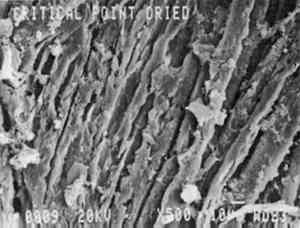
Fabric dried by critical point procedures appears more clean and less entangled than the previous two samples (fig. 4). Although yarn crowns display flattening in some areas, the fabric structure is readily apparent. There are some small areas of crusty deposit. It is possible that the solutions used in the drying process may
Most damaging to the appearance of the fiber and fabric structure is the vacuum freeze-drying treatment. As can be seen in figure 6, the surface of the yarns and fibers are covered with a crust of amorphous material that obscures their appearance so that the characteristic structures of the linen fabric cannot be seen. The overall fabric appearance shows considerably more alteration than that seen in the air dried and critical point-dried samples. The cause of this great extent of encrustation lies either in the freezing of the water or in the application of a vacuum in this treatment. In freezing water, the formation of the water crystals may have pushed the contaminants out of the fiber onto the front of the ice structures being formed. On the other hand, the vacuum may have drawn the contaminants to the surface of the fibers.
Comparison of the vacuum freeze-dried sample to the sample that was slowly dried in the freezer (fig. 7) reveals that the latter treatment did not produce large amorphous encrustations. Although some fiber entanglement is seen, the fabric structure is readily apparent. The fiber surfaces of frozen and slowly dried fibers are clean and display few encrustations. They are comparable in appearance to both the critical point-dried and air-dried material. It seems apparent, then, that the vacuum applied in the vacuum freeze-drying procedure is sufficient to draw the contaminants out of the fibers and create deleterious results.
A handkerchief that had been dried slowly in the freezer was examined for comparative purposes. The fabric structure of the handkerchief is readily apparent (fig. 8). This is a fine plain-woven
The satisfactory visual results obtained through slow drying in the freezer support that choice as appropriate in the drying of waterlogged textiles. While air drying is often the method of choice simply because the conservator's laboratory is not equipped for anything else, if there is question concerning the identity and condition of the fibers, the effects of air drying would be unknown. Freezing appears to be a preferred choice when there is an immediate need to preserve textile items from deterioration, as would be the case when there is a large quantity of waterlogged material and the individual pieces cannot be assessed and conserved immediately. The waterlogged material can be dried with no apparent damage to fiber structure; degradation by any mechanism is effectively eliminated. Beyond air drying, slow drying in a freezer compares favorably with the other techniques investigated. Freezers are also widely available and cost less than some of the equipment needed for other drying methods. In the event of disasters with water, emergency freezing facilities have been devised. Koesterer and Geating (1976) report that a walk-in thermal chamber designed for spacecraft testing was used to vacuum freeze dry books that had been soaked with water in the quenching of a library fire. Access to a freezer facility at the Ohio State University allowed the rapid freezing of the large quantity of textiles salvaged from the trunk. The cost of renting freezer space was minimal; the greatest expense was the construction of framed screens to support the garments. Most freeze-dry units used in research are small because they rely on vacuum pumps to obtain sufficiently low pressure while maintaining sufficiently low temperature. Larger units are available with larger more costly vacuum pumps. Although the equipment can be expensive, operation is inexpensive, requiring only the cost of the cooling fluid to maintain the low chamber temperatures. Due to the process of fluid exchange in the critical-point dryer, the maximum size of commercially available equipment is approximately 0.5 ft3 or smaller, most critical-point dryers are much smaller. Units could be made to larger dimensions, but with concomitant increasing cost since the chamber must withstand pressures of more than 1,200 psi. As mentioned, critical-point drying was examined for experimental purposes in this work to study differences in the structure of fibers dried by different methods. While critical-point drying is not feasible for large textile items, the comparable appearance of fibers after critical-point drying and after slow drying in the freezer supports the suitability of slow drying for waterlogged materials. Ethanol dehydration may be an attractive choice because it can be conducted in a standard laboratory with appropriate ventilation conditions and because the visual results obtained are satisfactory. However, it is less desirable because it may incur some change in the chemistry of the materials being treated. Freezing textiles in their natural state results in no chemical alteration of the fibers, whereas ethanol dehydration may dry fats or oils from some of the textiles. |