AN INVESTIGATION INTO THE REMOVAL OF ENZYMES FROM PAPER FOLLOWING CONSERVATION TREATMENTTHERESA MEYER ANDREWS, WILLIAM W. ANDREWS, & CATHLEEN BAKER
ABSTRACT—The efficiency of rinsing two different α-amylases out of paper after conservation treatment was measured using radioactively labeled enzymes. The radioactive enzymes were used to treat small samples of Whatman and Japanese papers. The papers were subjected to various rinsing procedures, and the radioactivity remaining in the paper samples was determined. Buffers were used to maintain the pH of the enzyme solutions, and their effect in the removability of the enzymes was analyzed. Two water rinses were shown to be sufficient in removing more than 99% of both enzymes tested under optimal conditions. It was also found that increasing the concentration of the enzyme solution resulted in an increase in retention of enzymes in the paper. 1 INTRODUCTIONEnzymes are often used in paper conservation to aid in the removal of adhesive residues from previous repairs or to facilitate removal of poor-quality secondary backing supports and mats.1 It is often necessary to remove these materials from paper artifacts because they may contribute to the deterioration of the paper as they age, or it may be desirable to return the object to its original format. In many cases, these extraneous materials cannot be safely removed by mechanical means, and the use of enzymes is required. The enzyme amylase is used to remove starch-based substrates such as pastes. Proteases and trypsins are used to remove protein-based substrates such as animal glues, gelatin, and casein.2 Various rinsing procedures are employed to remove the enzymes after treatment. It is important to reduce their quantity as much as possible because it is not known whether they may also be harmful if allowed to remain in the paper. The major thrust of research previously undertaken has focused on the application and use of various types of enzymes for treatment of artifacts in paper and painting conservation. The general procedure following an enzyme treatment has been to rinse the paper with room temperature water. In addition, some conservators employ an alcohol or hot water rinse in an attempt to denature any residual enzymes. Quantitative analysis has not yet been undertaken to determine the extent to which enzymes are removed from paper artifacts by rinsing alone. The aim of our research was to establish whether enzymes are in fact removed from paper after rinsing with water. The procedure involved tagging the enzymes with the radioactive iodine isotope, iodine 125, and determining if any radioactive enzyme was left in the paper after rinsing. 2 EXPERIMENTAL MATERIALS AND METHODSWe chose to concentrate on two α-amylases used on two very different types of papers commonly encountered by paper conservators. We selected a dense, wove, naturally aged, gelatin Because radioactive materials were being used, large sheets of paper that would more realistically resemble an artifact could not be used. Maneuvering radioactive papers of such a large size would have been impossible behind the lead shields and would have created large amounts of radioactive waste. The ratio of surface area of the experimental paper sample to the volume of radioactive enzyme solutions and subsequent rinses used was estimated using a large sheet of paper and appropriate volumes of solutions as models. The ratio used in the experiments was 1 ml of solution per sq cm of paper. Two α-amylases were chosen for the experiment. One was a highly purified bacterial enzyme from a Bacillus species (Sigma catalog no. A6380), and the other was a crude fungal enzyme preparation from Aspergillus oryzae (Sigma catalog no. A0273). These specific enzymes were chosen because they appear in conservation literature and represent a range of amylases from pure to crude. From information supplied by Sigma Chemical Company, the A6380 amylase had an activity of 2080 units/mg solid and the A0273 had an activity of 31 units/mg solid. Activity is an indication of how many units of the enzyme are found in the protein content of the solid. Both amylases are assayed at pH 6.9 at 20�C. An assay at this pH favors the A6380 amylase, as the pH is closer to its optimum of pH 6.0 than to the optimum of pH 5.0 for the A0273 amylase. We estimated that if we used the A6380 amylase at 1/10 the concentration of the A0273 amylase, the activities of both would be roughly the same.3 The enzymes were iodinated by the chloramine-T method illustrated in figure 1(Hunter and Greenwood 1962; Greenwood et al. 1963). This method specifically iodinates tyrosine residues in proteins forming a stable, covalent protein-125I bond. The method is generally accepted to be mild enough so as not to affect the activity of the protein being labeled, in this case the ability of the amylase to degrade starch. The details of the procedure are as follows: 0.5 millicurie (mCi) of Na125I (5 μl) was added to 5 μl of deionized H2O and 10 μl of 0.25 M sodium phosphate, pH 7.5. This solution was stirred in a polypropylene test tube, and the following solutions were added in sequence: 10 μl of 0.5 mg protein/ml amylase in 0.05 M sodium phosphate pH 7.5; 10 μl of 5 mg/ml chloramine-T in 0.05 M sodium phosphate pH 7.5; and 100 μl of 1.2 mg/ml sodium
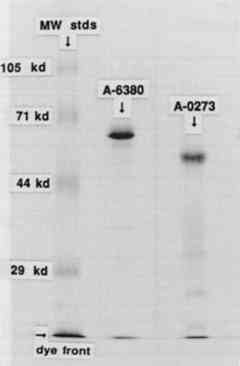
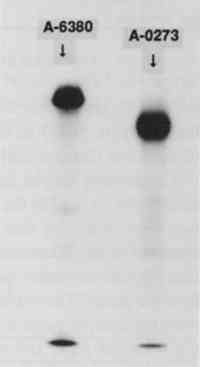
To separate unincorporated 125I from the iodinated protein a centrifuge column packed with Sephadex G-25 Fine was used after the method described by Penefsky (1979). Briefly, a 3 ml syringe was plugged with glass wool and filled with a slurry of Sephadex. This was allowed to drain, and the column was equilibrated with 10 ml of 0.05 M sodium phosphate, pH 7.5. The syringe column was then spun for 2 minutes in a swinging bucket clinical centrifuge to remove excess buffer from the column. The iodination mixture (0.24 ml) was applied to the top of the Sephadex bed5 and the column was again spun for 2 minutes at the same rpm. The eluate from the column contained the radioactive enzyme, while the unincorporated 125I was retained in the Sephadex bed. A small sample of the radioactive enzyme was added to 5 μg of the nonradioactive enzyme and was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel (Laemmli 1970). The gel was stained for protein by Coomassie blue, dried, and exposed to x-ray film overnight. Exposure of the paper samples to the radioactive enzyme was performed as follows: A nonradioactive solution of the enzyme was prepared, 0.1% (w/v) for the A6380 amylase and 1% (w/v) for the less pure A0273 amylase. The radioactive enzyme was added to this solution to make 100,000–700,000 cpm (counts per minute) per ml (generally 1 μl of the radioactive enzyme was added per ml of enzyme solution). This solution was thoroughly mixed, and 1 ml was dispensed onto each paper sample (1 square cm). The samples were exposed to this solution for 45 minutes, removed, touched briefly to filter paper, and put into the first rinse. Rinses were 2 ml each, and rinse time was 5 minutes for each rinse. The samples were briefly touched to filter paper between rinses. In all the experiments, the enzyme solutions and all the rinses were used at room temperature. Two controls were routinely performed: (1) a paper sample that was not rinsed, and (2) a sample that was rinsed on a Millipore suction device with 2 ml of buffer followed by 5 ml of deionized water. This latter control was considered to represent the most thorough rinsing possible. After rinsing, the samples were dried and counted in a Beckmann gamma counter for 125I. A portion of the original enzyme solution was also counted. The percentage of the radioactivity remaining on the paper was obtained by dividing the cpm on the paper after rinsing by the initial cpm per ml of enzyme solution. Percentages are listed in tables 1–3. TABLE 1 α-AMYLASE A6380 TABLE 2 α-AMYLASE A6380 TABLE 3 α-AMYLASE A0273 3 RESULTS AND DISCUSSION3.1 IODINATION OF α-AMYLASESThe SDS-PAGE gel of the iodinated amylases is shown in figure 2. This technique separates proteins on the basis of their molecular weight. The first lane, on the left, contains the molecular weight standards; the second lane contains the iodinated A6380 amylase; and the third lane contains the A0273 amylase. Both of the enzymes gave a major protein band upon staining. The less pure amylase contains fainter bands, indicating the presence of additional proteins in this preparation. According to the manufacturer, only about 25% of the less pure amylase is protein. An autoradiograph of the same gel can be seen in figure 3. The exposed dark bands on the autoradiograph indicate the presence of material that has been labeled by the radioactive iodine. Comparison of the stained gel with the autoradiograph shows that the
3.2 TREATMENT OF THE PAPER SAMPLES WITH IODINATED AMYLASESA total of eight experiments were conducted involving various rinsing procedures and various enzyme buffer solutions. Data from these experiments are compiled in tables 1–3. 3.2.1 α-Amylase A6380Table 1 contains data gathered on the more pure α-amylase, A6380. Initially the enzyme was used in a 10 mM sodium acetate buffer, pH 5. At the commencement of the experiment, we were not aware that 5 was not the optimum pH for A6380. This pH environment was selected because it was the pH recommended for α-amylase A0273 in conservation literature (Burgess and Charette 1981). The no-rinse samples show that 1–3% of the total cpm is retained in the paper samples. After two water rinses, less than 1% of the total cpm remained on the Whatman paper, whereas more than 2% of the total cpm remained on the Japanese paper. This was an unexpected result, since the Japanese paper is much less dense than the Whatman paper and was expected to rinse more thoroughly. Further water rinses did not remove appreciably more enzyme from either paper type. We reasoned that rinsing the paper first with the same buffer in which it was treated might help to keep the enzyme soluble during the A search of the biochemical literature on this amylase (Menzi et al. 1957; Ono et al. 1958) revealed its pH optimum to be 6 instead of 5, so we decided to try treating the paper with the enzyme at pH 6 in both a sodium acetate and a sodium phosphate buffer. Phosphate is a more effective buffer than acetate at this pH. The two buffers were tested at both 10 mM and 50 mM. A reduction in the amount of enzyme retained after rinsing was seen at this pH in both paper types. This reduction was particularly dramatic in the case of the Japanese paper, reducing the counts retained almost 20-fold. Use of either the 50 mM sodium phosphate or the 50 mM sodium acetate buffer at pH 6 resulted in the least amount of counts retained by either paper type. An additional experiment was performed with α-amylase A6380 to determine whether the binding of enzyme to the paper was due to the presence of substrate (starch) or an inherent property of the paper. The Whatman and Japanese papers, with and without a paste substrate, were treated with the enzyme in a 10 mM sodium acetate buffer, pH5. The results of this experiment are presented in table 2. The presence of the paste substrate on the papers did not significantly affect their retention of radioactivity, indicating that retention was an inherent property of the paper and not caused by the substrate. During this experiment the concentration of the enzyme was also varied to determine the effect of enzyme concentration on retention of 3.2.2 α-Amylase A0273The data obtained with the crude α-amylase A0273 are compiled in table 3. The published pH optimum is 5 (Matsubara et al. 1959), and all experiments were performed at this pH. The results for this enzyme confirm that four water rinses were no more efficient than two rinses in removing enzyme from the paper. The use of acetate buffer rinses followed by water rinses was again no more effective than rinsing with water alone. Again, adding a final ethanol rinse or using a suction rinse was no more effective in removing enzymes than water rinses alone. Raising the acetate concentration in the enzyme solution to 50 mM did not appreciably reduce the amount of enzyme bound to the Whatman paper. However, changing the buffer solution to 50 mM sodium phosphate reduced the amount of enzyme bound by 60%. It should be noted that with the crude enzyme approximately 75% of the solid weight of the preparation is not protein, at least according to the label on the bottle. Since the iodination labeled only the protein, the other 75% of the preparation is not labeled and therefore not detectable by these experiments. It was not necessarily a goal of these experiments to remove all of the starch substrate from the paper samples. However, when an iodine-potassium iodide reagent test for the presence of starch (Browning 1977) was performed on the paper samples following treatment, it was found for both enzymes that using the 50 mM phosphate buffer rather than acetate buffer with the enzyme was the most effective method for removing all the substrate within the given 45-minute period. 4 CONCLUSIONSIt is clear from the data that following rinsing, residual enzyme does remain in the Whatman paper in extremely small amounts, and how much seems to depend upon the purity (and concentration) of the enzyme: 0.5 μg of the pure amylase A6380/sq cm of paper versus 5 micrograms of the crude amylase A0273/sq cm of paper. Whether this residual enzyme is detrimental to the paper is not known and requires further experimentation. It would seem to be good practice, however, to remove as much of the enzyme and related materials as possible following conservation treatment. It is generally known that proteins tend to darken upon aging, and because enzymes are proteins, they may discolor if left behind in the paper. It is also possible that residual enzymes could become reactivated sometime following conservation treatment. For example, a reactivated residual amylase might cause the reversal of a repair, hinge or lining if starch was the adhesive used. The conditions that might cause such a reversal have not been examined, but it seems expedient to recommend as thorough a removal of the enzyme as possible. The data also suggest that the retention of enzyme by the paper appears to be an inherent property of the paper and is not necessarily caused by the presence of substrate on the paper. In addition, the amount of enzyme retained by the paper increased as the enzyme concentration increased. This finding suggests that the minimum amount of enzyme should be used when treating a paper artifact. Following this experiment, it was discovered that the enzyme A6380 can be used in extremely low concentrations, for example, 0.028% for an immersion treatment or 0.28% for local application The data further suggest that sodium phosphate, not sodium acetate as mentioned in some conservation literature, is a more effective buffer for the tested enzymes both in the removal of substrate and the removal of the enzymes from the paper. At the present time, we can recommend, with some reservation, the use of a sodium phosphate buffer to buffer the enzyme solution or as a rinse. Any deleterious effect that this buffer may have on the aging properties of papers has yet to be determined. Nevertheless, if a porous paper is being treated, the use of a sodium phosphate buffer might be warranted to significantly reduce the amount of enzyme left behind. While the results from these experiments using two amylases seem to indicate that simple water rinsing is efficacious, it is not known whether these data are generally applicable to the other common enzymes used in paper conservation such as protease and trypsin. Similar experiments using these enzymes should be performed to determine the most effective rinsing procedures. It is quite possible that in the case of the Whatman paper the gelatin surface-sizing helped to seal the paper from the amylase while the kizukishi did not have any such protection. If a protease is used on a protein surface-sized paper (or amylase on a starch surface-sized paper), the results might more closely resemble the ones obtained for the kizukishi. Presumably this result occurs because the enzyme can more easily get into an open paper structure but for some reason has a hard time getting out. The differences in the results obtained for the Whatman and kizukishi papers may not depend wholly upon the existence of a surface size; they may be more closely related to the structure of these two very different papers. The Whatman paper is comprised of cotton fiber, probably chemically processed with a strong alkaline solution, perhaps bleached, mechanically beaten to yield relatively short fibers, and formed into a sheet that is quite compact and dense. The kizukishi, on the other hand, is made up of a bast fiber from the inner bark of a shrub. Chemical processing was probably limited to a mild wood ash cook with no chemical bleaching, hand beating, and formation into a thin, rather open sheet with the long fibers essentially intact. It is also possible that the neri (a natural mucilaginous formation aid, e.g. tororo-aoi) used in the formation of the Japanese paper may have some effect on the attraction and retention of the amylase in this experiment. These are very interesting questions, and obviously further research is needed. But the conclusions that can be reached from this research can be summarized as follows:
Judging from the results of these experiments, it does not seem advantageous to use a denaturant such as alcohol for a routine rinse. Although hot water, as either a rinse or a denaturant, was not tested, its use as a rinse would also seem to be redundant given the test results. It might still be advisable, however, to reserve these two techniques for an emergency, to stop an enzyme treatment immediately for example. Pretesting the paper and media with any of the solutions under consideration including ACKNOWLEDGEMENTSWe gratefully acknowledge Marie Louise Hammarskj�ld and David Rekosh for allowing all the radioactive aspects of this investigation to be conducted in their laboratory facilities at the State University College of Buffalo, under the guidance of William W. Andrews. We also thank Robert Futernick for giving us the idea for this investigation and Debra Evans for her editing contributions. This research was carried out in spring 1990 by Theresa Meyer Andrews, under supervision of the other authors, as a partial requirement for the degree of master of arts and certificate of advanced study in art conservation. It was partially supported by a student award from the Faculty of Arts and Humanities, State University College at Buffalo. NOTES1. Enzymes are complex proteins produced by living cells that catalyze specific biochemical reactions usually at body temperature (98.6�F or 37�C). Enzymes are usually sold in powder form and are obtained from animal, plant (particularly fungal), and bacteriological sources. In addition to their source designation, enzymes are also described in terms of unit definition, activity, and purity. 2. The α-amylases attack randomly along the amylose chain (the major component of wheat starch) to convert it into dextrins (short-chained polysaccharides), which are very water soluble. The β-amylases attack amylose only from one end at a time and are therefore much less effective compared to α-amylases. Diastases contain both α- and β-amylases and, therefore, are not as effective as the α-amylases alone. Proteases cleave the bonds of proteins leading to shorter chain polypeptides and finally to amino acid molecules, which are water soluble. Trypsins, which come from the ferments of pancreatic juices, reduce proteins to water soluble peptones. 3. The methods used to define units, or in other words to assay the enzyme, are based on standardized reactions of enzymes with compounds that are not dealt with in conservation. It should not be assumed that the conditions used in the assay (e.g., pH, temperature) and given in a catalog or on the enzyme bottle label are the optimum conditions for that enzyme. To determine the optimum pH for a specific enzyme, it is necessary to consult the enzyme literature. 4. It is important to note that the pH of an enzyme solution has a direct bearing on its effectiveness. Most enzymes have optimum efficiency at a specific pH. An enzyme solution, not buffered to maintain the optimum pH, might still be effective, but the required action would occur very slowly, perhaps appearing not to work at all. In order to control the pH carefully at the required optimum, buffers are often used. Common buffers to control the pH of enzyme solutions include sodium phosphate and sodium acetate, but conservators have been somewhat reluctant to use them because these buffers might leave deleterious residues. 5. The correct rpm setting for any particular centrifuge can be ascertained by spinning trial columns with a mixture of blue dextran and potassium ferricyanide. The optimal rpm is the minimum that allows the dextran (blue) to elute from the column while retaining the ferricyanide (yellow) on the column. In general, 1,000 rpm is sufficient. 6. The purity of an enzyme is based on two things: (1) the percentage of protein (i.e., enzyme(s)), in the solid, and (2) the homogeneity of the protein fraction (the presence of one or more enzymes). The designation “crude” therefore could indicate that there is not much protein in the solid and/or that the protein fraction is not homogeneous. Generally enzymes described as “crude” have not been recommended for use in paper conservation, but a crude enzyme does not necessarily mean that it cannot be used. Of particular importance to paper conservators is whether any other enzymes present in a crude preparation include undesirable ones, such as cellulase (or other cellulose-digesting enzymes). Unfortunately, at the present time it is not possible for a conservator to SOURCES OF MATERIALSEnglish Paper, Cotton Fiber with Gelatin and Alum, Watermark:J Whatman 1891 Private collectionEnzyme, α-amylase: From Bacillus species, A6380, Type II-AEnzyme, α-amylase: fungal, crude, from Aspergillus oryzae, A0273, Type X-ASigma Chemical Co., St. Louis, Missouri 63178 Japanese paper, Kizukishi, 100% Kozo,Japanese precipitated wheat starch, Zin shofu, 15% Paste after cooking, diluted for useConservations Materials Ltd., Sparks, Nevada 89431 REFERENCESBaker, C.1991. Enzymes: Description and use in paper conservation. Unpublished course handout. Browning, B. L.1977. Analysis of paper. New York: Marcel Dekker, Inc.83. Burgess, H., and C. L.Charette. 1981. Aspects of image safety in the use of enzymes in paper conservation. In ICOM preprints, 6th Triennial MeetingOttawa. 81/14/10. Greenwood, R. C., W. M.Hunter, and J. S.Glover. 1963. The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochemical Journal89:114–23. Hunter, W. M., and R. C.Greenwood. 1962. Preparation of iodine-131 labelled human growth hormone of high specific radioactivity. Nature194:495–96. Laemmli, U. K.1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature227:680–85. Matsubara, S., T.Ikenaka, and S.Akabori. 1959. Studies on Taka-amylase A-VI. On the α-maltosidase activity of Taka-amylase A. Journal of Biochemistry (Tokyo) 46:425. Menzi, R., E. A.Stein, and E. H.Fischer. 1957. Propri�t�s de deux α-amylases de B. subtilis. Helvetica Chimica Acta40:534. Ono, S., K.Hiromi, and Y.Yoshikawa. 1958. Kinetics of hydrolic reaction catalyzed by crystalline bacterial α-amylase-I. The influence of pH. Bulletin of the Chemical Society (Japan) 31:957. Penefsky, H. S.1979. Methods in enzymology. New York: Academic Press. 56:527–30. OTHER SOURCESBolton, A. E.1977. Radioiodination techniques. Arlington Heights, Ill.: Amersham Corp. Boyer, P., ed., 1960. The enzymes, 2d ed.New York: Academic Press. 4:338. Cooper, D., C.King, and J.Segal. 1987. The use of enzymes in partially non-aqueous media. In Conservation of library and archive materials and the graphic arts, ed.G.Petherbridge. London: Butterworths. 25–29. DeSantis, P.1983. Some observations on the use of protease in paper conservation. Journal of the American Institute for Conservation23:7–27. Grattan, D., J. St.Hilaire, H.Burgess, and J.McCawley. 1987. The characterization of enzymes for use in paper conservation. In Conservation of library and archive materials and the graphic arts, ed.G.Petherbridge. London: Butterworths. 15–24.
Wanser, H.1990. The unveiling of A. J. Downing's plan for Washington, D.C., 1851. American Institute Wendlebo, ⊘., and B.Fosse. 1970. Protein surgery. Restaurator1(4):245–48. AUTHOR INFORMATIONTHERESA MEYER ANDREWS is currently an NEA advanced intern in paper and photographs conservation at the San Francisco Museum of Modern Art. She has an M.A. and Certificate of Advanced Study from the Art Conservation Department at the State University College at Buffalo. She has completed internships in both paper and photographs conservation at the California Palace of the Legion of Honor, the Metropolitan Museum of Art, the International Museum of Photography at George Eastman House, and with Jose Orraca. She has also worked with Debbie Hess Norris in her private practice and audited the photograph conservation block at the University of Delaware. Address: San Francisco Museum of Modern Art, Paper and Photographs Conservation, 401 Van Ness Avenue, San Francisco, Calif. 94102. WILLIAM W. ANDREWS is currently a scientist at Chiron Corporation in Emeryville, Calif. He received his Ph. D. in biochemistry from the University of California, San Diego. He has worked at Synbiotics Corporation in San Diego and was also a postdoctoral research associate at the University of Buffalo, Main Street Campus. Address: Chiron Corporation, Horton Street, Emeryville, Calif. 94608. CATHLEEN BAKER is associate professor of paper conservation in the art conservation department at the State University College at Buffalo. Before commencing her present teaching career in 1978, she was the paper restorer in charge of the Witt Collection of Drawings at the Courtauld Institute Galleries. She has an MA in art history from Syracuse University. She has given many conservation workshops, and was an instructor for the 1987 and 1991 ICCROM paper conservation courses. Address: Art Conservation Department RH 230, State University College at Buffalo, 1300 Elmwood Ave., Buffalo, N.Y. 14222.
 Section Index Section Index |