EFFECTS OF AQUEOUS LIGHT BLEACHING ON THE SUBSEQUENT AGING OF PAPERTERRY TROSPER SCHAEFFER, MARY T. BAKER, VICTORIA BLYTH-HILL, & DIANNE VAN DER REYDEN
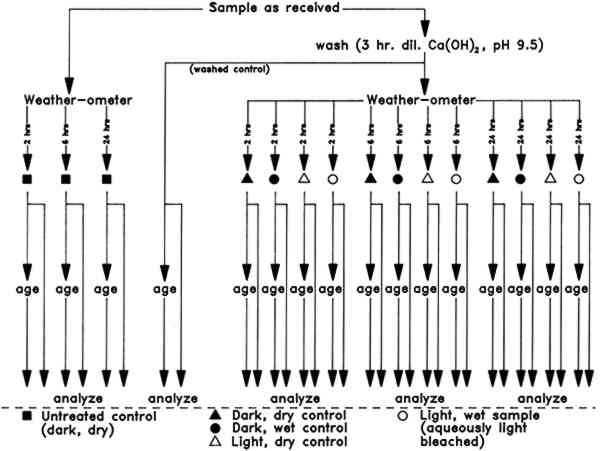
ABSTRACT—The effects of aqueous light bleaching of a naturally aged, 100% cotton rag, gelatin-sized paper on the subsequent aging of that paper have been investigated. Effects of exposure to various control conditions were compared with those due to aqueous light bleaching. Physical properties of all papers were monitored by measurement of surface pH, optical properties, stress-strain tensile behavior, and presence of sizing. Measurements were made before and after treatments and after artificial aging at 90�C and 50% RH for 20 days. An unsized filter paper, made from 100% cotton fibers, was exposed to identical conditions, and the same properties were measured. For the gelatin-sized paper, aqueous light bleaching resulted in less color reversion after artificial aging than did exposure to any of the control conditions. However, the sized paper specimens exhibited significant change in tensile properties. Similar responses were also observed in controls that were immersed in the dark. The changes noted are likely to be attributable to immersion temperature. All paper specimens were significantly embrittled by artificial aging. The changes in tensile properties of both types of papers upon aging did not appear to be influenced by exposure to light during the bleaching procedure. 1 INTRODUCTIONThe technique of aqueous light bleaching to diminish stains in paper was first brought to the attention of paper conservators in 1980 by Keyes at the International Conference on Conservation of Library and Archive Materials and the Graphic Arts in Cambridge, England. Simply stated, the technique utilizes exposure to light from the sun (e.g., van der Reyden 1981; Eldridge 1982 or an artificial light source such as fluorescent lamps (e.g., Branchick et al. 1982; Baker 1986), preferably with far ultraviolet radiation removed by filtration, to reduce discoloration in paper while the paper is submerged in a bath of purified and buffered water. Many variations in the procedure have been used, such as the “moisturizing sandwich” (Keyes 1980). However, specific requirements for successful application of aqueous light bleaching have been found to be: prebathing the object, using a buffered or slightly alkaline immersion solution, and restricting treatment to low lignin content paper. Because the procedure is straightforward and an effective tool, many paper conservators have incorporated it into their stain removal repertoire. Their reasons for using this bleaching method are many: (1) It is relatively easy to control; (2) the color of the paper is quite natural after treatment, as opposed to the sometimes overly bright white of chemical bleaching; (3) the paper feels stronger afterward; and (4) the procedure avoids the introduction of another extraneous chemical into an already degraded paper. The chromophores in discolored paper may be degradation products of cellulose or sizing, or they may have been formed by interactions among these chemicals or between them and other inclusions or impurities in the paper, such The possible disadvantages of aqueous light bleaching, which are similar to some of those incurred with other bleaching treatments, are that the absorbed light energy may: (1) cause formation of colored products in the components of the paper; (2) catalyze formation of colorless degradation products in the exposed paper, which may degrade further to colored compounds upon subsequent aging (Burgess 1988); and (3) induce degradation of the paper compounds, which could result in changes in mechanical properties. Several investigators have addressed the above-mentioned possibilities, studying the immediate effects of aqueous light bleaching on the properties of various new and old papers. The surface strength of some papers appears to be increased (van der Reyden 1981), while the tensile strength appears to remain unchanged (van der Reyden et al. 1988); colored substances will appear in some types of papers (Savard 1986); increased efficiency of the treatment may or may not be related to an increase of the pH of the solution(Eldridge 1982); and changes in degree of polymerization (DP) of cellulose after treatment appear to be indetectable (LePage and Perron 1986; Lee et al. n.d.). The effects of the aqueous light bleaching treatment on the subsequent aging of paper have received less attention (Savard 1986; Lienardy and van Damme 1989; Hofmann et al. 1991; Flieder et al. 1991; Lee et al. n.d.). Color reversion will occur, but the extent of this reversion compared to that of aged, unbleached control papers has not always been quantified. The mechanical properties of treated, aged papers have received little unequivocal documentation. Few studies have included aged papers likely to have been used by artists, such as the 1956 protein-sized cotton paper used in this study. An earlier study performed at the Conservation Analytical Laboratory, Smithsonian Institution (CAL) was undertaken to determine to what extent various aspects of an aqueous light-bleaching treatment would affect the color and tensile properties of certain papers (van der Reyden et al. 1988). In these experiments a naturally aged, mixed pulp, alum rosin-sized paper and unsized mixed pulp and cotton linter papers were aqueously light bleached or subjected to various control conditions (e.g., dark immersed or exposed to light while dry) for up to 96 hours in a Weather-ometer. Measurements of paper color before and after treatments showed that the aqueous light bleaching conditions were by far the most effective in removing discoloration from the mixed pulp papers and that the greatest decrease in discoloration occurred in the first 2 hours under these experimental conditions. Interestingly, neither aqueous light bleaching nor dark immersion, even at the elevated temperatures reached in the Weather-ometer, had a significant effect on The aqueous light bleaching research project at CAL has now been extended to include a study of the effects of accelerated aging on color and tensile properties of two papers that represent a modern control paper and a naturally aged artists' paper. As in the previous study, the papers were aqueously light bleached, or subjected to various control conditions, in the Weather-ometer (fig. 1). Physical and chemical properties were monitored before and after the exposures and after artificial, humid oven, aging.
The results of this study, indicate that aqueous light bleaching is an effective method for decreasing discoloration in the papers used and also that less color reversion occurs upon artificial aging of samples which received the aqueous light-bleaching treatment as compared to papers subjected to various control conditions. However, potentially disadvantageous alterations in other properties of the papers did occur. These changes did not appear to be directly attributable to light exposure, although they suggest that further detailed study of the conditions for treatment should be undertaken before aqueous light bleaching can be employed without reservation as a conservation technique. 2 MATERIALS AND METHODS2.1 PAPERSThe papers used in this study were:
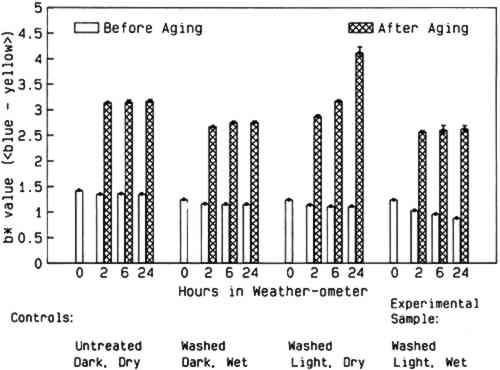
Analytical procedures, including spot tests, optical and scanning electron microscopy The information obtained from analyses of W1 and W56 papers “as received” (untreated) and after washing confirmed the manufacturer's specifications. Elemental analysis of the untreated W56 paper by EDX documented the presence of aluminum and sulfur. After washing, the presence of these elements was barely detectable. The observations are consistent with removal of a major portion of the alum and other aluminum salts during the washing step. Scanning electron micrographs of washed papers showed a significant increase in the cross-sectional area of most fiber lumens. Greater variation in interfiber spaces was also evident. Both these observations may be related to the ca. 15% increase observed in nominal thickness of the washed W56 paper, from 0.0165 � 0.0007 cm before to 0.0196 � 0.0005 cm after washing (n = 5). 2.2 EXPERIMENTAL DESIGNAll the W1 and W56 papers were handled as works of art on paper would be, with the additional precaution that plastic gloves were always worn when the papers had to be manipulated by hand. The overall experimental protocol is diagrammed in figure. 1. Details of the procedures are given in the appendix. 2.2.1 Washing and paper preparationA piece of each paper was reserved as an untreated control. Other sheets of the W56 and W1 papers were washed by soaking them individually in a Ca(OH)2 solution, initial pH 9.5, and then drying them between blotters, felts, and Plexiglas under weight. This preliminary step was undertaken to simulate the usual conservation procedure of washing a discolored paper object in an alkaline bath before bleaching as well as to provide washed controls to isolate the effects of alkaline water alone. The untreated papers and the washed papers were cut into specimens of appropriate size for exposure to aqueous light bleaching or the other, control, conditions described below. 2.2.2 Aqueous light bleaching and control exposure conditionsAqueous light bleaching was carried out in an Atlas Ci35 Weather-ometer, with each individual paper specimen suspended in a 600 ml flat-sided polystyrene culture bottle as described previously (van der Reyden et al. 1988). Thirty such bottles could be placed in the Weather-ometer chamber at one time, permitting 15 specimens of W1 paper and 15 specimens of W56 paper to be exposed simultaneously. In practice, 3 specimens of each paper were exposed to the same conditions, but for three different time periods: 2, 6, or 24 hours. Thus, five different experimental conditions were used for each of the two papers (see fig. 1). These were: (1) unwashed control paper, kept dry in the dark; (2) washed control paper kept dry in the dark; (3) washed control paper immersed in Ca(OH)2, initial pH 9.5, in the dark; (4) washed control paper kept dry and exposed to light; and (5) washed sample paper immersed in Ca(OH)2, initial pH 9.5, and exposed to light, which is equivalent to aqueous light bleaching. All dark One specimen of each paper subjected to each experimental condition was removed from the Weather-ometer in its bottle after each of the specified exposure times. The immersed papers were blotted and dried in a felt and Plexiglas press under weight. After the incubations the pH of all—and the temperatures and protein content of some—immersion solutions was measured. Details of these procedures are included in the appendix. The entire experiment was performed twice. 2.2.3 Artificial agingAll papers that had been in the Weather-ometer were cut in half. One set of these halves was reserved for analysis. The other set was sewn into Plexiglas frames for humid oven aging. They were artificially aged for 20 days in the dark at 90�C and 50% RH in an Associated Environmental Systems HK-4116 temperature-humidity chamber. These conditions have been used previously for artificial aging studies (Lee et al. 1989b; Erhardt et al. 1987). Washed papers that had not been in the Weather-ometer were aged at the same time. Aged papers were subsequently analyzed using the same procedures as those applied to the un-aged portions of each specimen. 2.3 EVALUATION OF EFFECTS OF TREATMENTS AND AGING ON PAPER PROPERTIESIn addition to the qualitative chemical analyses described in section 2.1, each paper specimen was characterized physically by surface pH, colorimetry, and relaxation tensometry. Experimental details of these analytical procedures are provided in the appendix. In this study, changes in surface pH have been regarded as a qualitative indicator of changes in the acidity of the paper. Because of the recognized inaccuracy of the measurement, only changes of at least one-half pH unit are considered significant. Colorimetry was used as a quantitative, objective measure of paper appearance. Reflectances throughout the visible range were recorded, and CIE L∗a∗b∗ values were generated from these. Comparisons of these values for different specimens will indicate not only changes in lightness-darkness, but also shifts in red-green and yellow-blue color coordinates.2 The CIE L∗la∗b∗ data for all papers are given in table 1 and table 3. TABLE 1 COLORIMETRIC DATA FOR WHATMAN 1 PAPER TABLE 3 COLORIMETERIC DATA FOR WHATMAN 1956 PAPER Tensile data obtained with sensitive relaxation tensometers were used to monitor mechanical properties of the papers before and after treatments and artificial aging. To use these data, nominal stress (force on the paper strip divided by cross-sectional area of the strip) was plotted as a function of strain (percent change in length). In these graphs, the relative strength of the paper is indicated by the stress withstood at a given strain; the slope of the curve is an indication of the stiffness; and decreased strain at failure implies increased brittleness of the paper. A summary of tensile data for all papers (ultimate stresses and strains to break) is presented in table 2 and table 4. TABLE 2 ULTIMATE STRESS AND MAXIMUM STRAIN SUSTAINED BY WHATMAN 1 SAMPLES TABLE 4 ULTIMATE STRESS AND MAXIMUM STRAIN SUSTAINED BY WHATMAN 1956 SAMPLES 3 RESULTS3.1 WHATMAN 1 (UNSIZED CONTROL PAPER)3.1.1 Morphological and chemical characterizationThe thickness of W1 paper was not significantly changed by washing, by aqueous light bleaching, or by incubation under any of the other conditions to which it was subjected. Some treated papers that had been used for measurement of tensile properties were also examined 3.1.2 Surface pHThe pH of the untreated W1 control paper was 6.9 � 0.1, and that of the washed control paper was pH 6.5 � 0.1. The surface pH of the W1 papers was significantly decreased by Weather-ometer treatment, typically by 0.3 to 0.7 units. The specimens most severely affected were those exposed to light while dry. The pH of the immersion solutions fell, as expected. All those that had remained in the dark fell to 6.7 � 0.1; the immersion solutions that had been exposed to light had a pH of 6.3 � 0.2. Humid oven aging lowered the surface pH of all specimens significantly. Least affected were the papers that had not been washed before Weather-ometer treatments. Their surface pH following aging was in the range 5.5–5.7. In contrast, the surface pH's of the washed W1 papers were all in the range 4.7–5.1 after exposures in the Weather-ometer and artificial aging, regardless of the particular conditions to which they were exposed. The papers exposed to light appeared systematically to have pH's at the low end of this range. 3.1.3 ColorimetryThe colorimetry data for all W1 papers are presented in table 1. The untreated W1 filter paper did not appear discolored to the eye. Washing this paper did not have a significant effect on its appearance. Brightness and green-red color values were unchanged, and only a slight decrease in b∗ occurred (decrease in yellow chromophores) (fig. 2). Weather-ometer exposures of washed specimens caused further slight decrease in b∗, with the largest changes occurring in the aqueously light-bleached samples. The extent of this change appears to have been correlated to time of aqueous light bleaching.
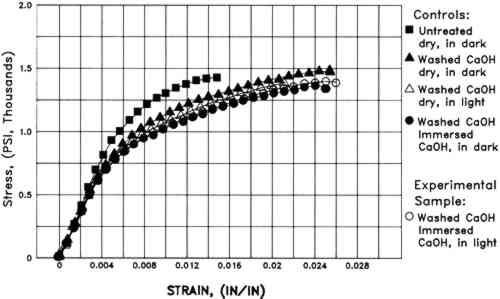
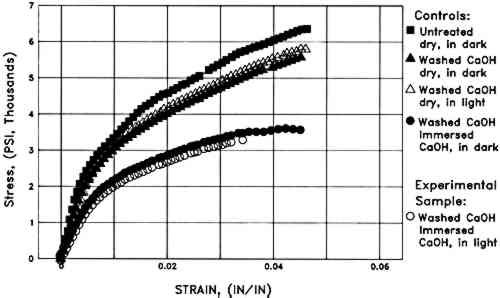
Humid oven aging caused a slight darkening of all the W1 papers (lower L∗). Changes in the aqueously light bleached samples are not distinguishable from those in washed controls that were immersed in the dark in the Weather-ometer (table 1). However, exposure of the W1 paper to light while dry resulted in significantly more discoloration upon subsequent humid oven aging (fig. 2). Increases in both red and yellow absorption (increases in a∗ and b∗) were directly related to time of dry light exposure in the Weather-ometer (table 1). 3.1.4 Tensile MeasurementsWashing the unsized W1 paper caused a significant increase in maximum strain (1.9 � 0.2% vs. 2.5 � 0.2%) but no statistically significant change in stiffness or ultimate stress to break. Immersion and/or exposure of W1 paper to light in the Weather-ometer caused no statistically significant changes in stress to break (table 2). There were no obvious systematic changes in brittleness due to Weather-ometer exposures. The averaged data (n = 3) for each of the papers exposed for 2 hours to each of the different incubation conditions are shown in figure 3. Aside from the obvious increase in maximum strain of all the washed papers, the tensile characteristics of the specimens can be seen to be very similar despite the disparate exposure conditions they endured.
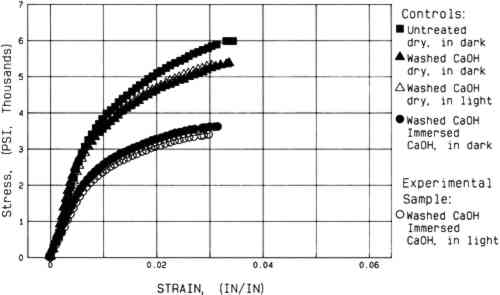
Artificial aging caused marked embrittlement but no significant changes in stress to break of the washed paper specimens (fig 4). In addition
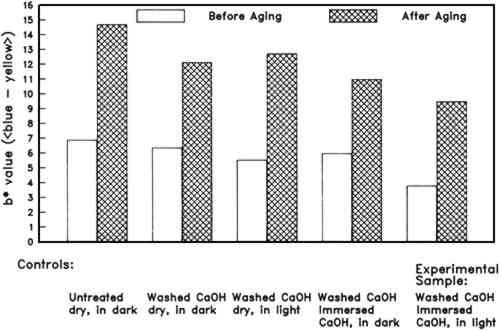
3.2 WHATMAN 1956 (ALUM- AND GELATIN-SIZED ARTISTS' PAPER)3.2.1 Morphological and chemical characterizationAll papers on which spot tests were performed gave positive results for both the alum and the ninhydrin protein test. However, W56 paper that had been immersed in the Weather-ometer for 24 hours responded to the ninhydrin test with blurred spots and slower color development, which might be indicative of lesser amounts of protein present. The characteristic protein bands were still present in an FTIR reflectance spectrum of a paper that had been immersed while in the Weather-ometer. Loss of some protein from the W56 paper into the immersion solution was confirmed by Lowry assays of two W56 immersion solutions for protein, using a gelatin protein standard. Both a light and a dark immersion solution had ca 0.25–0.3 mg protein per ml of solution. This result corresponds to a loss of 15–18 mg of gelatin from the paper specimen, or about 6–8% of the weight of the paper before incubation in the Weather-ometer. Very low levels of aluminum were also detectable by EDX in the papers after they had been in the Weather-ometer, whether or not they had been immersed. This result is consistent with the positive alum spot tests obtained on all the differently treated papers that were tested. The thickness of the W56 paper increased by approximately 15% after washing, a result that may be due to swelling of the paper as a consequence of removal of size. Immersion during incubation in the Weather-ometer did not cause any additional change in the thickness of W56 papers beyond that observed after the washing step. Artificial aging also had a negligible effect on paper thickness. 3.2.2 Surface pHSurface pH of the untreated W56 paper was 4.7 � 0.1. The washing procedure raised the surface pH to 5.3 � 0.1. The surface pH of the papers after Weather-ometer treatments did not significantly change. The pH of the immersion solutions fell about 1 unit further than the corresponding W1 immersion solutions did. The extent of decrease was somewhat greater for the aqueous light-bleached samples than for those kept in the dark. It appeared to depend slightly on the length of time in the Weather-ometer. The surface pH of all washed, aged W56 papers was in the range 4.5–4.7. The value appeared to be independent of type or length of Weather-ometer exposure. The surface pH of all the W56 controls aged without washing or without Weather-ometer exposure was decreased to 4.5 by humid oven aging. 3.2.3 ColorimetryThe CIE L∗a∗b∗ values for all the W56 papers are summarized in table 3. The W56 paper appeared cream colored when received. It was lightened slightly by washing in dilute Ca(OH)2, losing some red- and to a lesser extent, yellow-absorbing material. Immersion and light exposure in the Weather-ometer both caused additional color loss and lightening of the papers. Aqueous light bleaching resulted in a synergistic effect, seen most markedly in the time-dependent decrease in b∗. For example, immersion in the dark or dry light exposure for 2 hours each caused about a 15% decrease in b∗,
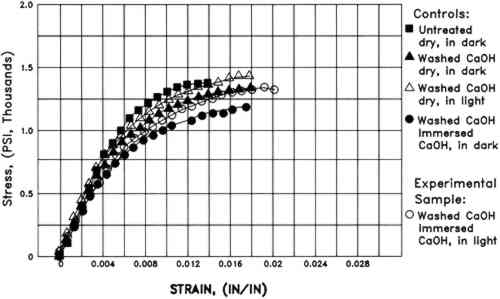
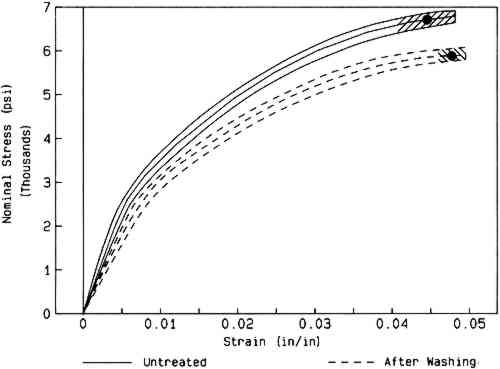
Marked color reversion occurred in all W56 papers upon humid oven aging (table 3). The extent of darkening and discoloration depended strongly on the conditions of Weather-ometer incubation. After artificial aging, only the papers that had been immersed remained significantly lighter than the washed control. The samples that were aqueously light bleached for 24 hours were the least discolored by aging. Again, the changes in the amount of yellow-absorbing material were the most dramatic (fig. 5). Of all the washed, Weather-ometer–incubated W56 papers, those that were exposed to the light while dry showed the greatest color reversion. In addition, these were the only papers in which a statistically significant difference between recto and verso color was observed for b∗, and they also had markedly more red-absorbing material. They did not, however, reach the extent of discoloration that the unwashed, untreated W56 paper did upon humid oven aging (table 3). 3.2.4 Tensile measurementsThe tensile behavior of the W56 paper as received (untreated) and after washing is shown in figure 6. The washed paper appears to display a slight decrease in stress to break, compared to the unwashed controls. However, the ca. 15% increase in thickness of the W56 paper after the washing step (see above) would contribute substantially to the apparent decrease in nominal stress, which is inversely proportional to the cross-sectional area (= width � thickness) of the paper strip.
The W56 controls that were exposed to dry Weather-ometer conditions did not display significant changes in their tensile properties,
4 DISCUSSIONThe results of this investigation demonstrate that aqueous light bleaching decreases discoloration immediately after treatment of both the sized and unsized papers. In addition, the extent of color reversion caused by humid oven aging of these papers is significantly less than that caused by aging controls that had been exposed to light while dry. After aging, the aqueously light-bleached W56 paper remained less discolored than the controls that were immersed and kept in the dark during Weather-ometer incubation. The W56 cotton rag paper used in this study is a very strong paper that is also very deformable. Its strength is undoubtedly due in part to the presence of gelatin sizing.3 The disadvantageous effect of the aqueous light-bleaching treatment for the W56 paper was the marked decrease in its stress to break; however, similar decreases occurred in controls that were immersed in the dark during Weather-ometer incubation. This result suggests that the reduction in stress to break was caused by immersion of the paper for a second time and it was not directly owing to a photochemical reaction. The removal of some of the gelatin sizing could have contributed to this result; the paper lost some of its reinforcement with the size and also may have swelled as a result, causing an increase in cross-sectional area and a subsequent apparent decrease in stress to break. Two factors might have enhanced protein solubilization during Weather-ometer incubation. Concomitant loss of alum during the washing step may have softened the originally hardened gelatin, so that it was more easily dissolved out of the paper during the next immersion. Second, the unavoidably elevated temperatures in the Weather-ometer chamber (see appendix) would have increased protein solubility. Additional experiments to determine the effect of temperature on this process are under consideration. It should be noted that in an early study of aqueous light bleaching (Branchick et al. 1982), the samples that suffered the greatest loss of strength were 18th-century rag papers incubated at the highest temperature used in the investigation (40.5�C). The reduced strain to break of the W56 papers that were immersed during Weather-ometer incubation may also be due in part to loss of gelatin size. The difference between the strain to break of the 2 hour light- and dark-immersed papers could be attributable to a The surface pH of the W56 paper was increased slightly by washing, but it fell back approximately to its original value upon artificial aging. The surface pH of the W1 paper was lowered slightly by the initial wash, and lowered much further by artificial aging. These observations suggest that the dilute Ca(OH)2 bath did not adequately neutralize acidic moieties in the paper and did not provide a buffer reserve that could successfully counteract acid groups formed during aging.4 The conditions to which the papers were exposed in the Weather-ometer were rather extreme. The W56 papers survived exposure to these conditions in surprisingly good shape. That the strong light itself—as distinct from immersion—was not responsible for significant deleterious changes in W56 papers that had been immersed is encouraging. Furthermore, no significant detrimental effect of light was discovered upon humid oven aging of the W56 papers immersed during Weather-ometer incubation. All papers suffered color reversion and embrittlement, but in no case was an aqueously light-bleached sample significantly more damaged by the artificial aging process than the corresponding control paper that was kept in the dark under otherwise identical conditions. The results of this study indicate that aqueous light bleaching decreases the extent of discoloration on subsequent aging of the W56 paper, even for the shortest time of exposure under the present experimental conditions. The reduced color reversion in aqueously light bleached W56 samples might be partly attributed to removal of gelatin size, which could discolor upon artificial aging. However, this loss is unlikely to account for all of the decreased color reversion since the controls immersed in the dark lost about the same amount of gelatin as those that were aqueously light bleached, yet the former developed significantly more yellow chromophores during humid oven aging than did the latter samples. It is interesting to note that the aqueously light-bleached W1 samples discolored the least after aging, discoloring less even than untreated papers. These results, for a paper composed solely of cellulose fibers, combined with the above results for sized W56, suggest not only that aqueous light bleaching removes chromophores from the paper directly but also that immersion during light exposure prevents colorless but potentially deleterious moieties from being present in the paper following that exposure. Whether this result is achieved by prevention of formation of the precursors that discolor upon aging or by removal of them into the immersion solution must be determined by more detailed chemical studies. That sizing, and the chemical composition of the paper fibers themselves, will have a major effect on the response of any paper to every step of the aqueous light-bleaching process cannot be overemphasized. For example, the above results demonstrate that the effects of immersion on naturally aged, protein-sized papers must be If loss of sizing is experimentally controlled, more subtle effects of light upon the mechanical properties of the paper may become evident. For documentation of these types of changes, chemical analyses and measurement of degree of polymerization are expected to be appropriate (Burgess 1985). It will also be desirable to use as a starting material a paper that is well defined chemically—that is, a new paper—and to discolor it by preaging it artificially under controlled conditions before subjecting it to further experimentation (Hofmann et al. 1991). Once the effects of the aqueous light-bleaching procedure in the long as well as the short term have been thoroughly documented, those papers for which it would be an efficacious conservation treatment can be identified, and appropriate treatment conditions can be determined. ACKNOWLEDGEMENTSWe thank Lambertus van Zelst and Alan Postlethwaite for providing the opportunity to continue this research project at the Conservation Analytical Laboratory, Marion F. Mecklenburg for advice and discussion, and Melanie Feather for expert technical guidance in SEM/EDX data acquisition. This project could not have been completed in the limited time available without the assistance of paper conservation interns at the Conservation Analytical Laboratory, most notably Nancy McRaney and Danielle Nguyen. Emily Klayman, Crista Hofmann, Olga Souza, and Andrew Robb also participated. Terry Trosper Schaeffer thanks the Chemistry Department, California State University—Northridge, for the use of its Beckman DU-64 spectrophotometer, and the staff of the Conservation Analytical Laboratory for their cheerful hospitality. APPENDIX1 APPENDIX1.1 PAPER PREPARATIONA 14� � 21 in sheet of paper was misted with deionized water and submerged in a bath of 21 liters of Ca(OH)2 solution, initial pH 9.5, prepared by dropwise addition of a filtered, saturated Ca(OH)2 solution to deionized water until the desired pH was reached. The reverse osmosis water purification system at CAL consistently provides water of resistivity ≥ 18 Mohm-cm. After 4 hours, the paper was removed from the bath on polyester webbing, blotted between blotters, and then placed between blotters in a felt and Plexiglas press under ca. 1 psi weight. The blotters were changed after 5, 20, 40, and 60 minutes. A final blotter change was made the next day. Two sheets of each paper were so washed. The pH of the bath at the end of the washing procedure was 6.5 for the W56 paper and 7.3 for the W1. Each sheet of paper remained in the press 2 weeks, when it was removed, covered with polyester webbing, and allowed to equilibrate in the laboratory atmosphere for 1 day before being cut into 4 � 4 � in specimens with the machine direction in the short dimension. Cotton threads were sewn into two corners about � in from the edges, so that each paper could Papers were suspended vertically in 600 ml polystyrene flat-sided culture bottles as described previously (van der Reyden et al. 1988), oriented so that the felt side of the paper would face the light source in the Weather-ometer. To each flask that contained a paper to be immersed during Weather-ometer incubation was added 580ml of a stock pH 9.5 Ca(OH)2 solution prepared as above. Enough stock solution was prepared immediately prior to loading the papers to be adequate for all immersed papers in an experiment. 2 AQUEOUS LIGHT-BLEACHING AND CONTROL TREATMENTSIrradiance in the Weather-ometer chamber was maintained constant at 0.35 w/m2. Quartz tubes were used for the water jacket, which serves as a cooling filter for the xenon arc lamp. The walls of the polystyrene culture bottles filtered out most of the ultraviolet radiation (van der Reyden et al. 1988). The lamp power and the temperature in the Weather-ometer chamber were monitored throughout the experiment. The lamp power fluctuated between 7.1 and 7.4 kW during operation. To prevent fogging by water condensation on the bottle surfaces, the Weather-ometer was operated without humidity in the chamber. Because the instrument has no refrigeration unit, it was not possible to keep the chamber temperature at room temperature while the xenon arc lamp was on. Dry bulb temperature in the chamber rose to 39 � 1�C within a few minutes of starting the lamp and stayed in that range throughout the experiment. Immediately upon removal from the chamber, the temperature of the solutions in the bottles wrapped in foil were about 1�C above the temperature of the chamber. The temperature of the solutions in bottles exposed to light was 47–48�C. The temperature inside the “dry” control bottles could not be measured accurately. It is assumed to have been approximately the same as the chamber temperature. The pH of the immersion solutions was measured when the solutions had cooled to approximately room temperature. As expected, the pH of all immersion solutions fell during incubations in the Weather-ometer. One W1 immersion solution and two W56 immersion solutions were assayed by a modified Lowry procedure to determine if protein had been solubilized from the W56 paper. The dry condition controls were placed in archival polyester sleeves immediately upon removal from the Weather-ometer and stored in a binder. As each immersed paper was removed from its bottle, it was placed on a thick blotter and turned over after about 1 minute. These papers were then placed individually between 7 � 9 in blotters in a Plexiglas press and weighted under less than 1 psi. The blotters were changed twice at 10 minute intervals. The wet papers were then placed between blotters and felts in a larger Plexiglas sandwich and more heavily weighted overnight (less than 1 psi). The blotters were changed once the next day, and these papers remained in the press for 2 weeks, after which the dry papers were transferred to polyester webbing, covered with a fresh blotter, and allowed to equilibrate with the laboratory atmosphere for 1 day. 3 ARTIFICIAL AGINGAll papers that had been in the Weather-ometer were cut in half along the machine direction, with a fresh scalpel blade, to give two pieces 4 � 2 � in. The half of each paper that was to be artificially aged was sewn with cotton thread into a Plexiglas frame. The halves were positioned with the machine direction vertical At the end of the aging period, the papers were equilibrated overnight, still in the Plexiglas frame, in the dark at room temperature. After being cut out of the frames, they were stored in polyester film enclosures. 4 ANALYTICAL PROCEDURES.The following tests were applied:
NOTES1. Dr. David Martin of Whatman Paper, Ltd., London, advised us that Whatman records showed that the paper was made of 100% cotton rag with alum, gelatin, and soap flakes added. Further details of the manufacture of this paper are no longer available. 2. A major advantage of collecting spectral data is that any of the color measurement scales can be used to describe the results. CIE L∗a∗b∗ is considered to be one of the more useful and sensitive scales; however, many researchers use % reflectance or K/S values at certain wavelengths. These data can be taken from the tabulated spectral data recorded on the HunterLab instrument. For example, for measurements of some color standards, the following results were obtained: The wavelength used for % reflectance and K/S was 457 (TAPPI Standard 452 os-77). In addition to its sensitivity in making color changes more evident, the spectral data give a complete picture of what is happening to the specimen. For example, the curves for the standard tiles are very similar at the 457 nm area, but the orange tile spectrum shows peaks at 510 and 620 nm, while the yellow-green tile spectrum shows peaks at 525, 600, and 675 nm. 3. Application of animal glue has also been shown to increase the strength of Japanese papers; for some of these papers this strength advantage is maintained upon artificial aging (Inaba and Sugisita 1986). 4. Although it provides a solution with greater neutralizing capacity, the common procedure of diluting a saturated Ca(OH)2 solution 1:1 with water was avoided because it can yield a solution of pH 12 initially and because it does not provide a reproducibly reliable buffer reserve. It is also likely to result in precipitation of CaCO3 during the 24 hour incubation, as far more CO2 is absorbed from the air. 5. Readers interested in more complete reflectance data or in brightness values can obtain these by contacting the authors at the Conservation Analytical Laboratory. 6. Details of the construction and use of these instruments may be obtained from the Conservation Analytical Laboratory. REFERENCESBaker, C.1986. The doublesided light bleaching bank. American Institute for Conservation Book and Paper Group Annual4:88–91. Branchick, T. J., K. M.Keyes, and F. C.Tahk. 1982. A study of the bleaching of naturally aged paper by artificial and natural light. AIC preprints, 10th Annual Meeting, American Institute for Conservation, Washington, D.C.29–39. Browning, B. L.1977. Analysis of paper, 2d ed.New York: Marcel Dekker. Chaps. 8–10. Burgess, H.D.1980. The colour reversion of paper after bleaching. In: Conservation of Library and Archive Materials and the Graphic Arts, ed.G.Petherbridge. London: Butterworths. 57–70. Burgess, H. D.1985. Gel permeation chromatography: Use in estimating the effect of water washing on the long-term stability of cellulosic fibers. In Historic textile and paper materials: Conservation and characterization, ed.H. L.Needles and S. H.Zeronian. Advances in Chemistry series 212, Washington, D.C.: American Chemical Society. 363–76. Burgess, H. D.1988. Practical considerations for conservation bleaching. Journal of the International Institute for Conservation–Canadian Group13:11–26. Eldridge, B. P.1982. A sun bleaching project. American Institute for Conservation Book and Paper Group postprints. 1:52–55. Erhardt, D., D.vonEndt, and W.Hopwood. 1987. The comparison of accelerated aging conditions through the analysis of extracts of artificially aged paper. AIC preprints, 15th Annual Meeting, American Institute for Conservation, Washington, D.C.43–55. Feller, R.L., S. B.Lee, and J.Bogaard. 1982. The darkening and bleaching of paper by various wavelengths in the visible and ultraviolet. American Institute for Conservation Book and Paper Group postprints. 1:65–83. Flieder, F., M., Leroy, J. C.Andreoli, and F.Leclerc. 1991. Comparative study of four paper bleaching methods. Presented at the International Federation of Library Associations International Preservation and Conservation Research Seminar, New York. Hofmann, C., D.van derReyden, and M.Baker. 1991. Comparison and evaluation of bleaching procedures: The effect of five bleaching methods on the optical and mechanical properties of new and aged cotton linter paper before and after accelerated aging. American Institute for Conservation Book and Paper Group Annual10: 109–27. Inaba, M., and R.Sugisita. 1986. Effect of Doza on the deterioration of Washi (Japanese paper). Scientific Papers on Japanese Antiquities and Art Crafts31:32–40. Keyes, K. M.1980. Alternatives to conventional methods of reducing discoloration in works of art on paper. In Conservation of Library and Archive Materials and the Graphic Arts, ed.G.Petherbridge. London: Butterworths. 49–55. Lee, S. B., J.Bogaard, and R. L.Feller. 1989a. Darkening of paper following exposure to visible and near-ultraviolet radiation. Journal of the American Institute for Conservation28:1–18.
Lee, S. B., J.Bogaard, and R. L.Feller. 1989b. Damaging effects of visible and near-ultraviolet radiation on paper. In Historic textile and paper materials II: Conservation and characterization, ed.S. H.Zeronian and H. L.Needles. Advances in Chemistry series 410. Lee, S. B., J.Bogaard, and R. L.Feller. n.d. Bleaching by light, I: Effect of pH on the bleaching or darkening of papers in the dry and in the immersed condition under visible and near-ultraviolet radiation. In Symposium 88: The conservation of historic and artistic works on paper. Ottawa: Canadian Conservation Institute. Forthcoming. LePage, M., and J.Perron. 1986. Investigation of some aspects of the light bleaching of paper. Conservation Training Programs, 11th Annual Conference, University of Delaware, Winterthur, Del., 54–68. Lienardy, A., and P.vanDamme. 1989. R�sultats de recherches exp�rimentales sur le blanchiment du papier. Studies in Conservation34:123–36. Lowry, O. H., N. J.Rosebrough, A. L.Farr, and R. J.Randall. 1951. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry193:265–75. Mecklenburg, M. F.1984. The role of water on the strength of polymers and adhesives. Ph.D. diss., University of Maryland, College Park, Md. Phillips, G. O.1985. The effects of light on cellulosic systems. In Cellulose and its derivatives, ed.J. F.Kennedy et al. Chichester, England: Ellis Horwood. 119–34. Savard, G.1986. An investigation of whitening and colour reversion effects on light bleached, artificially aged paper. Conservation Training Programs, 12th Annual Conference, New York University and Columbia University, New York. 57–83. Schaeffer, T. T., M. T.Baker, and D.van derReyden. 1991. Effect of aging on an aqueously light-bleached, mixed pulp paper. American Institute for Conservation Book and Paper Group Annual10:205–15. van derReyden, D.1981. Wax pick testing: A preliminary study. Art Conservation Training Programs, 8th Annual Conference, New York University, New York. 62–73. van derReyden, D., M.Mecklenburg, M.Baker, and M.Hamill. 1988. Update on current research into aqueous light bleaching at the Conservation Analytical Laboratory. American Institute for Conservation Book and Paper Group annual7:73–106. AUTHOR INFORMATIONTERRY TROSPER SCHAEFFER received a B.A. in physics and biophysics (1961) and a Ph.D. in biophysics (1967) at University of California, Berkeley. Most of her research has been in the areas of interaction of photosynthetic pigments with other plant and bacterial membrane components, and ion transport across mammalian cell membranes. In 1989–90, she entered the field of conservation research when she was a Fellow-by-Courtesy, G.W.C. Whiting School of Engineering, Johns Hopkins University, and a Visiting Research Collaborator, Conservation Analytical Laboratory. She is a fellow in Paper Conservation Reseach at the Conservation Center of the Los Angeles County Museum of Art. Address: Conservation Center, Los Angeles County Museum of Art, 5905 Wilshire Blvd., Los Angeles, Calif. 90036. MARY T. BAKER received her B.S. in chemistry in 1980 and her Ph.D. in 1986 in materials science with a specialty in polymer science from the Institute of Materials Science at the University of Connecticut. She has worked at the Conservation Analytical Laboratory, Smithsonian Institution, as a research chemist since 1987, collaborating with conservators on projects such as the effects of fumigation on materials; treatment and characterization of coated papers; light bleaching of paper; and methods development for analysis of microsamples of paints, varnishes, and other materials. Her current research is on the modern polymeric materials in air and space artifacts, their aging mechanisms, storage, treatment, and display. Address: Conservation Analytical Laboratory, Museum Support Center, Smithsonian Institution, Washington, D.C. 20560. VICTORIA BLYTH-HILL is the senior paper conservator in the Conservation Center of the Los Angeles County Museum of Art. She is past chair of the Book and Paper Group of the AIC and has been a Fellow of that organization since 1988. She has presented lectures at AIC and other professional organizations and at universities on subjects ranging DIANNE VAN DER REYDEN received certificates in conservation from Harvard University Art Museums (1981) and the Conservation Center, Institute of Fine Arts, New York University (1980), along with an M.A. in art history (1979), serving internships at the Fogg Art Museum, the Library of Congress, and the Museum of Modern Art. She is senior paper conservator and co-head of the Paper Conservation Laboratory at the Conservation Analytical Laboratory, Smithsonian Institution, engaged in research in aqueous light bleaching and the effects of solvents on specialty papers and in the training of interns and professionals. She recently served as secretary of AIC and has been a Fellow for several years. Address: Conservation Analytical Laboratory, Museum Support Center, Smithsonian Institution, Washington, D.C. 20560.
 Section Index Section Index |