DYE ANALYSIS OF PRE-COLUMBIAN PERUVIAN TEXTILES WITH HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY AND DIODE-ARRAY DETECTIONJAN WOUTERS, & NOEMI ROSARIO-CHIRINOS
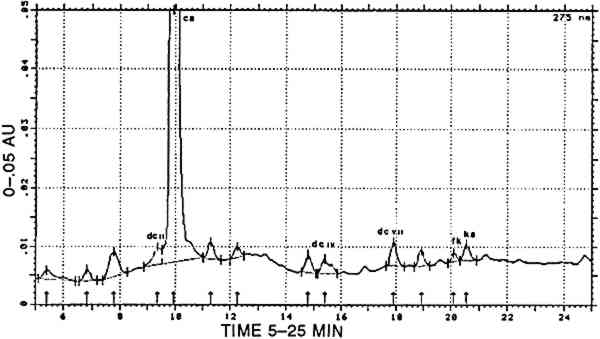
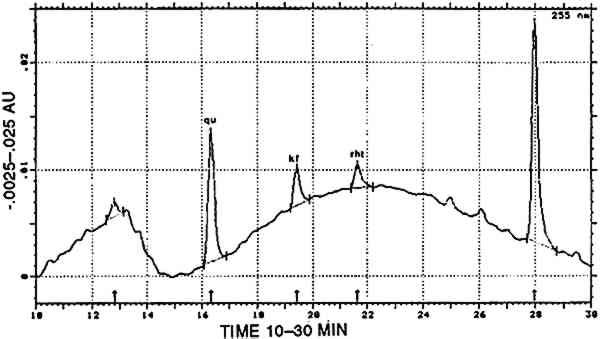
4 RESULTS4.1 REFERENCE SAMPLESAll analytical data are presented in table 2. The analyses of Dactylopius coccus insects from various origins and of other scale insect dyes was described in detail in previous papers (Wouters and Verhecken 1989a, 1989b). All compositions of cochineal dyeings from Zumb�hl (1979) conform to the data in Wouters and Verhecken (1989a). A dyeing with either Relbunium ciliatum or Galium (species not specified) is represented by one single sample in Zumb�h. According to the predominance of alizarin, Galium should be the source. Roots from Relbunium hypocarpium were obtained from the Royal Botanical Garden (Meise, Belgium). The dyeing of alum-mordanted wool was carried out by the procedure described by Zumb�hl, but the ratio roots/wool was 4/1 instead of 1/1. Both Relbunium dyes contain mainly purpurin, minor amounts of munjistin, and traces of other anthraquinoid products, among which rubiadin could be identified in Relbunium ciliatum. TABLE 2 CHEMICAL COMPOSITION OF REFERENCE DYEINGS The dye from Alnus jorullensis contained important amounts of luteolin, quercetin, and morin, making it rather unusual in the family of the yellow flavonoid dyes. The same is true for Salvia sagitata, which shows high amounts of apigenin and luteolin. Baccaris genistelloides contains three luteolin-like products: lu4, lu6, and lu8, each more abundant than luteolin. The most complex composition was found with Bidens andicola, in which, apart from luteolin as the main component, apigenin, lu1, lu2, luS, fisetin, and an unknown auronic component also occurred. Thus completely different luteolin-like compounds were present in either Baccaris genistelloides or Bidens andicola. Plant species that are supposed to contain tannin material have ellagic acid as the main component, accompanied by traces of quercetin and kampferol. These analytical features are unlikely to form a basis for species determinations with the aid of dye compositions. The Indigofera anil sample was analyzed in a different way. First, a pyridin extract (20 minutes at 100�C; (a) in table 2) was chromatographed according to Wouters and Verhecken (n.d.). Second, the usual acid hydrolysis procedure for mordant dyes was applied 4.2 UNKNOWN SAMPLESFrom 42 textile pieces, 157 samples were taken. Thirty turned out to be naturally colored fiber, and 11 remained unidentified. A first survey of the analytical results evidenced the predominant presence of well-known compounds, which formed the basis for a classification into several main groups. A more detailed consideration of the analytical results for each group enabled the creation of several sub-groups 4.2.1 Main group cochineal [COCH]Carminic acid was found in 46 samples. A typical cochineal analysis is presented in figure 1.
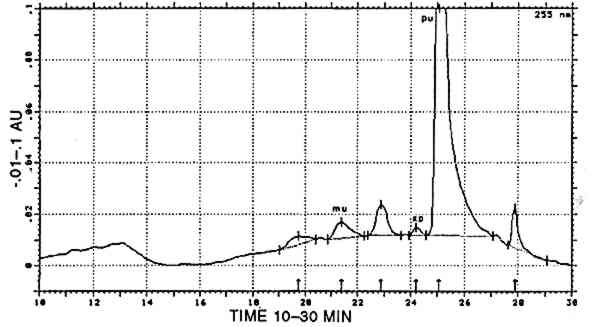
In 16 samples, carminic acid was the only compound that could be characterized. In 13 other samples, carminic acid was accompanied by one or more of the following products: dcII, dcIV, dcVII, flavokermesic acid, and kermesic acid. The relative ratios of dcII, carminic acid, flavokermesic acid, and kermesic acid conform to the data in Wouters and Verhecken (1989a) and to the composition of the reference samples. All 29 dyes are therefore considered to be pure cochineal, prepared from the scale insect Dactylopius coccus, and are designated [COCH]. In 2 samples, carminic acid was present together with purpurin; according to the composition of appropriate reference samples, this mixture represents a combination of cochineal with a vegetal dye from Relbunium. These dyes form the subgroup [COCH-RELB]. The carminic acid/purpurin ratio was totally different in these two samples (1/9 in the first, 7/3 in the second). This variation means that a totally different ratio of insect and plant extracts was originally used for dyeing. However, the actual hue is brown in both cases. In 15 samples, carminic acid was found in combination with indigotin (identified in a separate experiment by reductive bleaching). Occasionally, indigotin was also detected in the acid hydrolysate of these samples; in only two cases indirubin was found as well, in concentrations of up to and for not more than 7% (at 288 nm) relative to indigotin. Any combination of carminic acid with a blue indigoid dyestuff, whatever its composition, is designated [COCH]+[INDI]. 4.2.2 Main group Relbunium [RELB]Purpurin was present in 20 samples. In 2 of them it was the only component detected. In another 7 samples the minor products found were munjistin (≤ 7% relative to purpurin) and xanthopurpurin (≤ 2% relative to purpurin). A representative analysis of this main group is presented in figure 2. Purpurin, pure or in combination with small amounts of munjistin (table 2), surely represents a species from the Relbunium genus and is therefore represented by [RELB].
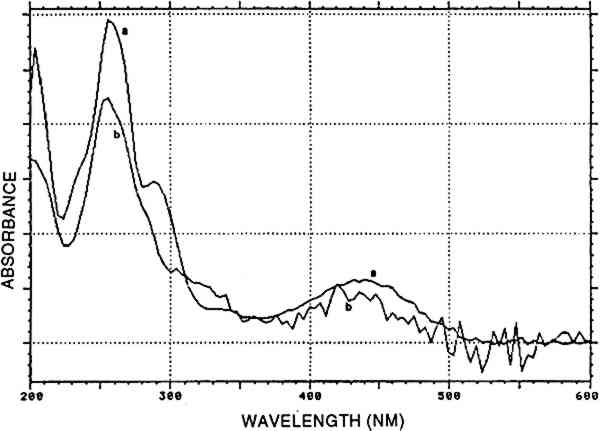
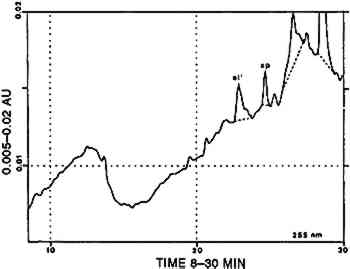
In one sample, munjistin was the most prominent component besides traces of purpurin Combinations with cochineal are referred to as [COCH-RELB] (see sec. 4.2.1) and those with yellow dyes containing luteolin as [LUTE-RELE] (see sec. 4.2.4). Indigotin was also found in five samples (identified by reductive bleaching), all showing both indigotin and indirubin in the acid hydrolysates. In all these samples, indirubin prevailed over indigotin. Any combination of purpurin with blue indigoid dyes is referred to as [RELB]+[INDI]. 4.2.3 Main Group Xanthopurpurin [XAPU]The anthraquinone xanthopurpurin (or purpuroxanthin) is reported to be present in the roots of Rubia tinctorum, R. cordifolia, R. sikkimensis, several Galium species, Asperula odorata, and Morinda umbellata(Thomson 1971). None of these plants occurs in the South American biosphere. Relbunium species, which do occur there, were not reported to contain xanthopurpurin, but mostly purpurin, munjistin, and pseudopurpurin (Fester and Lexow 1943b; Schweppe 1986; Thomson 1971; table 2). Four samples evidenced the presence of xanthopurpurin, with no trace of purpurin but accompanied by an anthraquinone-like dye, spectrally resembling alizarin (fig. 3) and in relative ratios ranging from 67/33 to 80/20 (alizarin-like/xanthopurpurin). A typical chromatogram is presented in figure 4. Several possible interpretations may be given to this phenomenon: Xanthopurpurin may be formed from purpurin by reduction (Sch�tzenberger 1865); however, aging processes mostly involve oxidation reactions, and the same should have occurred with other similar samples that still show purpurin as the most prominent compound (see main group [RELB]). The composition of [XAPU] may also refer to a specific Relbunium species not previously analyzed. Finally, a plant not belonging to the Relbunium genus might be involved. Further research on the plants themselves will be necessary to solve this problem, but the present analytical data as such seem interesting, especially when the historical and geographical context related to [XAPU] is considered (table 6).
TABLE 6 CORRELATION BETWEEN CULTURAL AND HISTORICAL DATA AND DYESTUFF GROUPS Three [XAPU] samples showed a combination with indigotin (identified by reductive bleaching). Only one of them showed appreciable amounts of indirubin in the appropriate acid hydrolysate. All three are designated as [XAPU]+[INDI]. 4.2.4 Main group luteolin [LUTE]Twenty-six textile samples contained luteolin and/or a series of products that spectrally closely resemble luteolin. Some of these so-called luteolin-like products were also found on modern wool samples dyed with actual Peruvian dye plants (Zumb�hl 1979; table 2). All data concerning these luteolin-like products are given in table 4. Although the differences in the relative retention times of some of these compounds are sometimes very small, different products are indeed involved, since lu3, lu5, lu6, lu7, lu8, and lu9 occur as distinct peaks in the same analysis. Figure 5 is a combination of two chromatograms made to visualize the relative position of each lu-like component. UV-VIS spectra of all these products are given in figure 6. TABLE 4 OCCURRENCE AND CHARACTERIZATION OF LUTEOLIN-LIKE COMPONENTS PRESENT IN THE DYES OF [LUTE] Just two analyses showed luteolin only, due to the small dye recovery that made accompanying products undetectable. Eight samples consisted of luteolin and lu1, with no further addition; in seven samples the relative ratios ranged from 13/87 to 23/77 (lu1/lu), calculated at 255 nm. This very reproducible relative ratio Another subgroup of three samples is characterized by the presence of 5–15% fisetin together with luteolin (always the main component) and lu1: [LUTE-FISE]. Fisetin is known to be a component of the dye prepared from Cotinus coggygria, but its main yellow compound is the accompanying 3′,4′,6-trihydroxyaurone (Wouters and Florquin 1987). However, this aurone never appears in the Peruvian samples, although similar products definitely occur in dyeings with Bidens andicola(table 2). It would not be surprising, however, that aurones remain undetected in historical samples, since 3′,4′,6-trihydroxyaurone was proven to be the less lightfast component in Cotinus coggygria(Wouters and Florquin 1987). The plant source, responsible for the combination of luteolin and fisetin on yarns, was used twice with a vegetal source of the Relbunium type. Thus, a further subgroup [LUTE-RELB] is characterized by the presence of luteolin, fisetin, and purpurin (two samples), luteolin and purpurin each being the main component once. In four samples a combination of luteolin with apigenin was found in a ratio ranging from 95/5 to 87/13 (luteolin/apigenin), which is in very close agreement with the situation in European weld Reseda luteola(Wouters 1987). However, in the Peruvian samples, lu1, lu2, lu3, lu4, and lu7 also occurred in different combinations. This subgroup is called [LUTE-APIG]. A remarkable subgroup, [LUTX], was found to contain numerous luteolin-like products, but no luteolin, lu1, or lu2 is present (seven samples). Instead, lu3, lu5, lu7, and lu8 may each be the main component; lu6 and lu9 were also detected. Any combination of a dye from the [LUTE) group with a blue indigoid dye (identified by reductive bleaching) is referred to as [luteolin subgroup]+[INDI]. It may be emphasized that a combination with indigotin occurred only once in [LUT1], once in [LUT4], never in [LUTE-FISE] and always in [LUTX]. In this last subgroup, in six samples, comparable amounts of indigotin and indirubin were found in the appropriate acid hydrolysates. 4.2.5 Main group tannins [TANN]Two analyses show the presence of ellagic acid, derived from hydrolysable tannins by the acid hydrolysis recovery procedure. Some other compounds with spectral characteristics similar to those of ellagic acid were also found in minor quantities. 4.2.6 Main group quercetin [QUER]This flavonoid is known to be the main coloring principle of Quercus tinctoria, indigenous to North America. It occurs in the wood as the glucoside quercitrin, which is readily converted to quercetin by acid hydrolysis. Apart from this valuable dye source, quercetin also occurs in numerous plants, mostly accompanying other flavonoid dyes. Quercetin was found in four Peruvian samples, once combined with luteolin, once with kampferol and rhamnetin (fig.7). The combination of luteolin and quercetin is rare and occurs also in the modern dyeing with Alnus jorullensis(Zumb�hl 1979; table 2).
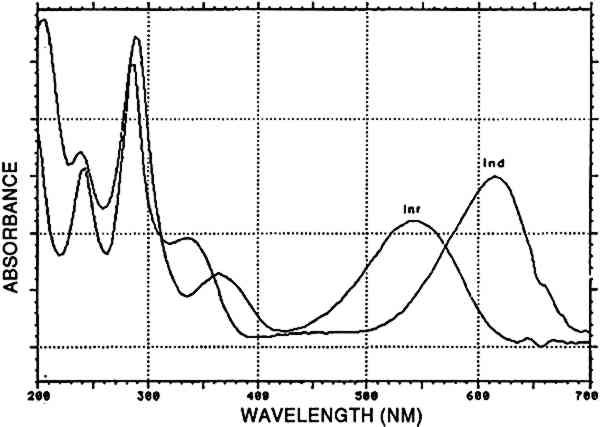
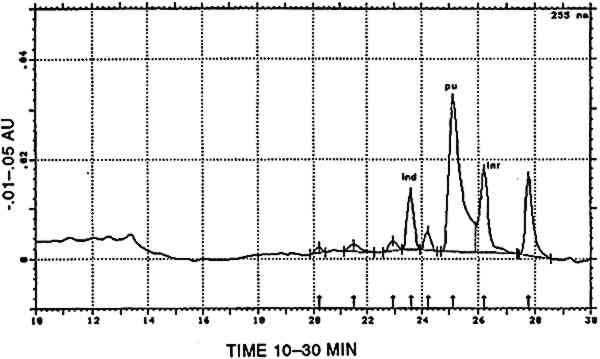
4.2.7 Main group indigotin [INDI]Indigotin detected by reductive bleaching (Hofenk-De Graaff 1974) as well as by HPLC of an acid hydrolysate is represented by the dye group [INDI]. The detection of indigotin and indirubin (fig. 8) in mixtures prepared to solubilize mordant dyes is probably due to the state of deterioration of the dyed fiber. When very deteriorated, the fiber disintegrates in the acid hydrolysis medium, and small indigoid particles may suspend in the liquid. Obviously, the higher solubility of indirubin in the acid hydrolysis medium may considerably alter the ratio between indigotin and indirubin (table 2). In the early Peruvian samples, indirubin was often more abundant than indigotin. The presence of varying amounts of indirubin in commercial indigo preparations was described by Popplewell Bloxam and Perkin (1910). High indirubin amounts may be obtained by manufacturing processes that favor the oxidation of indoxyl to isatin; hence, this feature may indicate particular methods of indigo dye preparation. An example of an analysis showing purpurin, indigotin, and indirubin is given in figure 9.
4.3 COMPILATION OF ANALYTICAL DATAIn table 5 are listed all the fabrics with their code numbers according to museum collection, hue of samples taken from them as judged by the naked eye, and type of dye groups found. Unknowns and naturally colored fibers were omitted. TABLE 5 COMPILATION OF ANALYTICAL DATA WITH REFERENCE TO EACH TEXTILE SAMPLE AND TO THE COLLECTION NUMBER OF EACH FABRIC |