DYE ANALYSIS OF PRE-COLUMBIAN PERUVIAN TEXTILES WITH HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY AND DIODE-ARRAY DETECTIONJAN WOUTERS, & NOEMI ROSARIO-CHIRINOS
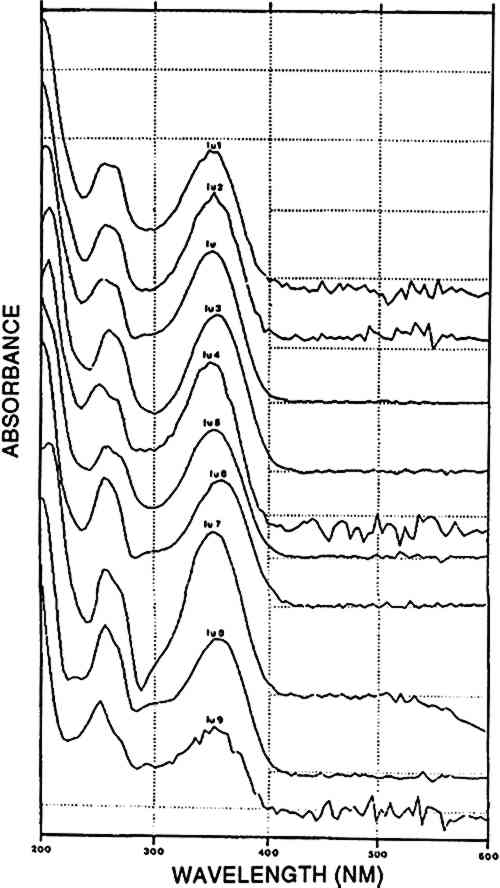
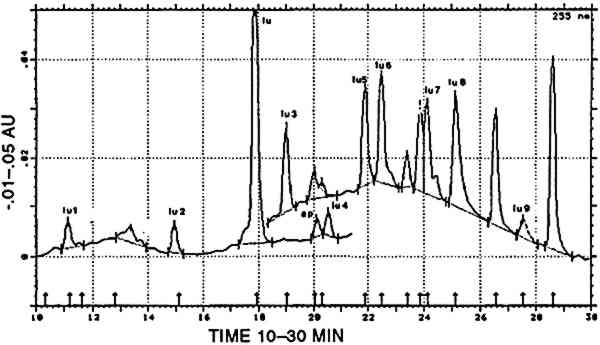
2 NATURAL SOURCES OF DYESThe substrates to be dyed were wool and cotton. Peruvian cotton, a type of Gossypium barbadense, exists in different hues from ivory to brown. The woolen fiber used was from the genus Llama, including the camelids llama, alpaca, vicuna, and guanaco; various natural color shădes of wool also exist. The natural colors of the fibers were used to enlarge the range of shades by combining them with dyes and dyed fibers (Fester 1940). The mordants and additives used in the dyeing process included several kinds of aluminium salts, derivatives of iron (e.g., black alcaparossa), plant ash, tannins, lime, urine, and vinegar (Fester 1940). A brief description of possible dye sources follows. For clarity, any dye known or thought to have been used in ancient Peru but not found in the present study is omitted from the discussion. 2.1 ANIMAL RED: COCHINEALThe most renowned natural source of cochineal dye is the scale insect Dactylopius coccus, which feeds on the cactus species Nopalea coccinellifera in Central and South America. Historical, geographical, and technical data on cochineal and other coccid insect dyes were reviewed by Verhecken (1988–89). The main dyestuff component is carminic acid, which may represent up to 20% by weight of the total insect body mass (Wouters and Verhecken 1989b). This high content of active dyestuff, together with the various beautiful bright shades that it may produce, made it a highly esteemed trading material in Central and South America. Within a few decades after its importation into Europe in the second quarter of the 16th century, it completely replaced similar insect dyes from local sources like Kermes vermilio and Porphyrophora polonica(Verhecken and Wouters 1988–89; Brunello 1973). Several other components were reported in the cochineal from Dactylopius coccus, the most important being flavokermesic acid and kermesic acid (Wouters and Verhecken 1989b). It is claimed that the typical Peruvian species Dactylopius confusus, which feeds on Opuntia exaltada and is known as “magno” or “macno,” was used to produce bright red shades on pre-Columbian Peruvian fabrics (Fester 1940; Verhecken and Wouters 1988–89; Donkin 1977; Yacovleff and Muelle 1934). Differences between the Mexican Dactylopius coccus and the Peruvian Dactylopius confusus have been reported, with special reference to the bluer hue of the former after ammoniacal treatment (Fester 1940). This difference in hue may be due to the bluish character of dcIII, a component detected in highly variable amounts in several Dactylopius coccus samples that is generated in ammoniacal medium and destroyed in acid (Wouters and Verhecken 1989a, 1989b). Since sufficient data to distinguish these two species from each other are not yet available, the dyes containing carminic acid as the main component will be referred to as cochineal. 2.2 VEGETAL RED: RUBIA, RELBUNIUM AND GALIUM SPECIESDyes of this type are located in the roots, between the outer skin and the woody heart, of various plants of the Rubiaceae family: Rubia tinctorum, R. peregrina, R. cordifolia, and R. munjista. The genus Relbunium also belongs to the Rubiaceae family, but species from this genus grow mainly in South America where the common European madder did not exist originally (Fester and Lexow 1943b). A typical Peruvian species is Relbunium microphyllum or R. chapichapi(Herrera 1939). Relbunium hypocarpium would have been the only species used in Chile, and neither its presence nor its use as a dye source in ancient Peru is certain (Antunez de Mayolo 1989; Fester and Lexow 1943b) The most important dyestuff components of Rubiaceae are alizarin, purpurin, munjistin, pseudopurpurin, xanthopurpurin, and rubiadin. The typical presence or absence of one or more of these compounds and the ratio between several of them indicate the original plant material that was used (Wouters 1985). In Relbunium, mainly xanthopurpurin, munjistin, purpurin, and pseudopurpurin would be expected (Thomson 1971). The absence of alizarin in Relbunium species is typical and represents a decisive difference between the common European madder (Rubia tinctorum) and the Galium species (Fester and Lexow 1943b; Thomson 1971). 2.3 VEGETAL YELLOWWeld, from the plant Reseda luteola, was the principal source of yellow dye in Europe. The predominant flavonoid components are luteolin and apigenin, at a ratio of approximately 9/1, measured at 255 nm (Wouters 1987). Antu�ez de Mayolo (1989) explicitly gives 15 plant species as sources for yellow dye. Three of these were used by Zumb�hl (1979): Alnus jorulliensis, Baccharis genistelloides, and Bidens andicola. Both luteolin and apigenin may be detected in these species as well as in Salvia sagitata a sample of which was present in the atlas of Zumb�hl (1979).In addition, other yellow dye components that are spectrally closely related to luteolin but that elute at totally different times are present in Baccaris genistelloides and Bidens andicola. Such compounds were also common in yellow pre-Columbian yarns, albeit at highly variable relative ratios. Since all these products will be considered further in this paper as “luteolin-like,” they will be designated by “lu,” followed by a number indicating their order of elution from the chromatography column. A list of all such products encountered in known and unknown samples is given in table 3; their spectra are shown in figure 6 and a representative chromatogram in figure 5. TABLE 3 GROUPING OF DYES ACCORDING TO THE PRESENCE OF SPECIFIC COMPOUNDS
2.4 VEGETAL BLUE: INDIGOThe coloring matter derived from Isatis tinctoria or Indigofera species is mainly indigotin. This dye is insoluble in water and must be transformed into a soluble derivative to be applied for dyeing. Cloth is dipped in the solution of this derivative and left to air dry, whereupon oxidation to the deep-blue indigotin occurs. This technique is called vat dyeing and involves physical precipitation of the water-insoluble dye onto the textile fibers. Much research on this type of dye remains to be done, owing mainly to the limited solubility of the dye in organic solvents and to its instability in these media (Kuramoto and Kitao 1979), which make separation techniques less applicable. Two greyish blue reference samples were available from Zumb�hl (1979): Indigofera anil and Cestrum hediondinum; Indigofera suffruticosa is mentioned by Antunez de Mayolo (1989). 2.5 TANNINSTannins occur in numerous plants and often in different plant morphological structures, e.g., quebracho from the wood of Schinopsis balansae and Schinopsis lorentzil, tara from the pods of Caesalpinia spinosa, and divi-divi from the pods of Caesalpinia coriaria. The first belongs to the group of condensed tannins that contain various catechin-like molecules and polymerize to phlobaphenes in acid. The latter two contain |