DYE ANALYSIS OF PRE-COLUMBIAN PERUVIAN TEXTILES WITH HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY AND DIODE-ARRAY DETECTIONJAN WOUTERS, & NOEMI ROSARIO-CHIRINOS
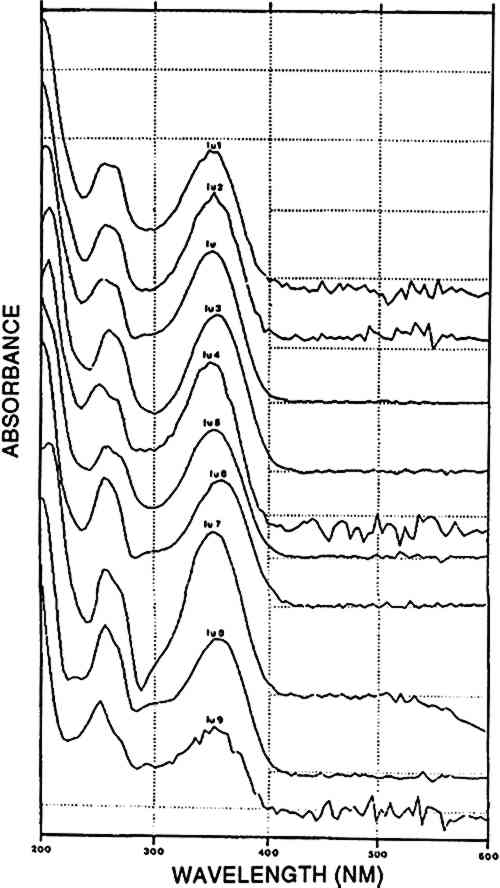
ABSTRACT—A series of textiles belonging to various pre-Columbian civilizations as well as a series of present-day Peruvian natural dyes were analyzed using high-performance liquid chromatography and diode-array detection. The analytical results were classified according to the composition of known dyes or to specific compositional patterns of unknown dyes. Plant reds (from Relbunium species) and animal reds (cochineal from the scale insect Dactylopius coccus) were each predominantly present before and after the Huari and Tiahuanaco cultural periods (700–1100 A.D.), respectively. During these periods both reds were found together. One red and one yellow vegetal dye were both found to occur in the Paracas necropolis only. The analysis of the complex yellow dyes may be useful for the determination of early Peruvian dyes. 1 INTRODUCTIONColor has always been an important element in the cultures of peoples all over the world. It was used not only to embellish an object or an individual but to indicate importance and hierarchical status. Some dyes or dyed textiles were even used to pay taxes (Donkin 1977; Born 1938; Baizerman 1985). The painstaking activities of searching for the natural sources of dyestuffs and selecting, extracting, and applying them form a sound basis for their high commercial importance. In present-day Peru, the technology used by the ancient Peruvians for the production of polychrome textiles has almost completely disappeared. A recent literature and field survey of dye plants traditionally used in Peru beginning in pre-Hispanic times, led to the taxonomic determination of 56 plant species (Antu�ez de Mayolo 1989). In another study, descriptions are given of dyeing procedures utilizing contemporary substrates, mordants, and dyes together with small samples of wool dyed by the procedures described (Zumb�hl 1979). In previous analytical dye studies various methods were used: comparisons of hues on yarns with the naked eye (Fester 1940); spectrophotometric analyses in the visible region (Fester 1940; Fester and Lexow 1943b; Saltzman 1978; Geiss-Mooney and Needles 1981); paper chromatography techniques (Gibaja Oviedo and Salazare de Cavero 1977); thin-layer chromatography (Schweppe 1986); and infrared spectroscopy (Martoglio et al. 1990). None of these methods involves quantitation of dye components or their spectral characterization. This type of data, however, represents an analytical refinement that might be expected to unravel the dye traditions for which there is sparse evidence from pre-Columbian Peru, e.g., regarding the existence of abundant sources for the production of yellow dyes (Antunez de Mayolo 1989; Martoglio et al. 1990). The study described in this paper uses high-performance liquid chromatography (HPLC) in combination with diode-array detection in the Forty-two fabrics were selected from the collections of the Royal Museum of Art and History (Brussels, Belgium) and the Museo Nacional de Arqueolog�a y Antropolog�a (Lima, Peru). The fabrics belong to different pre-Columbian cultures and to the Inca period, covering the era from 300 B.C. to 1532 A.D. The textile pieces were attributed to given cultural periods before any dye analysis was carried out, either by S. Purin of the Royal Museum of Art and History or by the staff of the textile department at the Museo Nacional de Arqueolog�a y Antropolog�a (see table 1) TABLE 1 HISTORY AND GEOGRAPHY OF PRE-COLUMBIAN CULTURES 2 NATURAL SOURCES OF DYESThe substrates to be dyed were wool and cotton. Peruvian cotton, a type of Gossypium barbadense, exists in different hues from ivory to brown. The woolen fiber used was from the genus Llama, including the camelids llama, alpaca, vicuna, and guanaco; various natural color shădes of wool also exist. The natural colors of the fibers were used to enlarge the range of shades by combining them with dyes and dyed fibers (Fester 1940). The mordants and additives used in the dyeing process included several kinds of aluminium salts, derivatives of iron (e.g., black alcaparossa), plant ash, tannins, lime, urine, and vinegar (Fester 1940). A brief description of possible dye sources follows. For clarity, any dye known or thought to have been used in ancient Peru but not found in the present study is omitted from the discussion. 2.1 ANIMAL RED: COCHINEALThe most renowned natural source of cochineal dye is the scale insect Dactylopius coccus, which feeds on the cactus species Nopalea coccinellifera in Central and South America. Historical, geographical, and technical data on cochineal and other coccid insect dyes were reviewed by Verhecken (1988–89). The main dyestuff component is carminic acid, which may represent up to 20% by weight of the total insect body mass (Wouters and Verhecken 1989b). This high content of active dyestuff, together with the various beautiful bright shades that it may produce, made it a highly esteemed trading material in Central and South America. Within a few decades after its importation into Europe in the second quarter of the 16th century, it completely replaced similar insect dyes from local sources like Kermes vermilio and Porphyrophora polonica(Verhecken and Wouters 1988–89; Brunello 1973). Several other components were reported in the cochineal from Dactylopius coccus, the most important being flavokermesic acid and kermesic acid (Wouters and Verhecken 1989b). It is claimed that the typical Peruvian species Dactylopius confusus, which feeds on Opuntia exaltada and is known as “magno” or “macno,” was used to produce bright red shades on pre-Columbian Peruvian fabrics (Fester 1940; Verhecken and Wouters 1988–89; Donkin 1977; Yacovleff and Muelle 1934). Differences between the Mexican Dactylopius coccus and the Peruvian Dactylopius confusus have been reported, with special reference to the bluer hue of the former after ammoniacal treatment (Fester 1940). This difference in hue may be due to the bluish character of dcIII, a component detected in highly variable amounts in several Dactylopius coccus samples that is generated in ammoniacal medium and destroyed in acid (Wouters and Verhecken 1989a, 1989b). Since sufficient data to distinguish these two species from each other are not yet available, the dyes containing carminic acid as the main component will be referred to as cochineal. 2.2 VEGETAL RED: RUBIA, RELBUNIUM AND GALIUM SPECIESDyes of this type are located in the roots, between the outer skin and the woody heart, of various plants of the Rubiaceae family: Rubia tinctorum, R. peregrina, R. cordifolia, and R. munjista. The genus Relbunium also belongs to the Rubiaceae family, but species from this genus grow mainly in South America where the common European madder did not exist originally (Fester and Lexow 1943b). A typical Peruvian species is Relbunium microphyllum or R. chapichapi(Herrera 1939). Relbunium hypocarpium would have been the only species used in Chile, and neither its presence nor its use as a dye source in ancient Peru is certain (Antunez de Mayolo 1989; Fester and Lexow 1943b) The most important dyestuff components of Rubiaceae are alizarin, purpurin, munjistin, pseudopurpurin, xanthopurpurin, and rubiadin. The typical presence or absence of one or more of these compounds and the ratio between several of them indicate the original plant material that was used (Wouters 1985). In Relbunium, mainly xanthopurpurin, munjistin, purpurin, and pseudopurpurin would be expected (Thomson 1971). The absence of alizarin in Relbunium species is typical and represents a decisive difference between the common European madder (Rubia tinctorum) and the Galium species (Fester and Lexow 1943b; Thomson 1971). 2.3 VEGETAL YELLOWWeld, from the plant Reseda luteola, was the principal source of yellow dye in Europe. The predominant flavonoid components are luteolin and apigenin, at a ratio of approximately 9/1, measured at 255 nm (Wouters 1987). Antu�ez de Mayolo (1989) explicitly gives 15 plant species as sources for yellow dye. Three of these were used by Zumb�hl (1979): Alnus jorulliensis, Baccharis genistelloides, and Bidens andicola. Both luteolin and apigenin may be detected in these species as well as in Salvia sagitata a sample of which was present in the atlas of Zumb�hl (1979).In addition, other yellow dye components that are spectrally closely related to luteolin but that elute at totally different times are present in Baccaris genistelloides and Bidens andicola. Such compounds were also common in yellow pre-Columbian yarns, albeit at highly variable relative ratios. Since all these products will be considered further in this paper as “luteolin-like,” they will be designated by “lu,” followed by a number indicating their order of elution from the chromatography column. A list of all such products encountered in known and unknown samples is given in table 3; their spectra are shown in figure 6 and a representative chromatogram in figure 5. TABLE 3 GROUPING OF DYES ACCORDING TO THE PRESENCE OF SPECIFIC COMPOUNDS
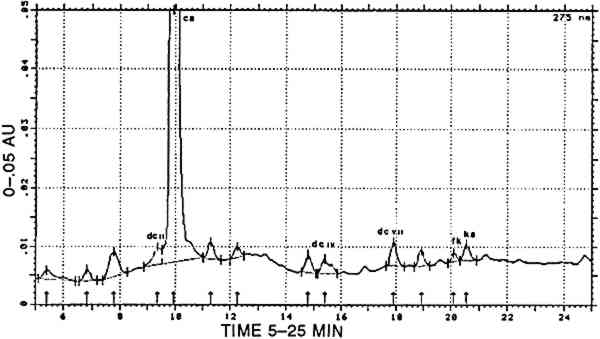
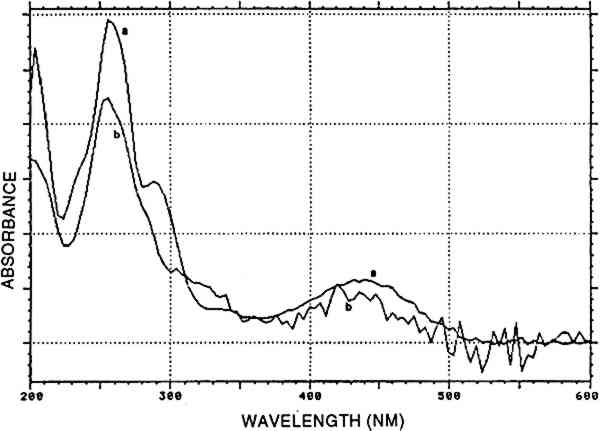
2.4 VEGETAL BLUE: INDIGOThe coloring matter derived from Isatis tinctoria or Indigofera species is mainly indigotin. This dye is insoluble in water and must be transformed into a soluble derivative to be applied for dyeing. Cloth is dipped in the solution of this derivative and left to air dry, whereupon oxidation to the deep-blue indigotin occurs. This technique is called vat dyeing and involves physical precipitation of the water-insoluble dye onto the textile fibers. Much research on this type of dye remains to be done, owing mainly to the limited solubility of the dye in organic solvents and to its instability in these media (Kuramoto and Kitao 1979), which make separation techniques less applicable. Two greyish blue reference samples were available from Zumb�hl (1979): Indigofera anil and Cestrum hediondinum; Indigofera suffruticosa is mentioned by Antunez de Mayolo (1989). 2.5 TANNINSTannins occur in numerous plants and often in different plant morphological structures, e.g., quebracho from the wood of Schinopsis balansae and Schinopsis lorentzil, tara from the pods of Caesalpinia spinosa, and divi-divi from the pods of Caesalpinia coriaria. The first belongs to the group of condensed tannins that contain various catechin-like molecules and polymerize to phlobaphenes in acid. The latter two contain 3 DYE ANALYSISSeveral factors may contribute to the actual hue of old textiles. Among the most important may be the chemical composition of the mordant and the aging processes, due to the action of electromagnetic radiation, cleaning activities, or the acidity of the atmosphere. Since these factors influence the hue observed over time, it will be difficult, if not impossible, to determine the natural source from observing the actual hue only. No natural dye is a pure product, and often the exact natural source of a given dye can only be derived from the presence of minor dye components. The most refined analytical result will be obtained if it is possible to consider all the dye components present on a dyed yarn and if their relative abundances may be calculated. Therefore, high-performance liquid chromatography (HPLC) was our method of choice. Great care was taken to perform any preliminary manipulation or the chromatography itself in a reproducible manner, so that the eventual transformations would be the same in reference and unknown materials. Only then it is relevant to compare analyses by considering relative ratios of dye components. 3.1 EXPERIMENTAL METHODAbout 1 mg of yarn was treated in 400 μl of H2O/methanol/37% HCl (1/1/2, v/v/v) for 10 min at 100�C in an open Pyrex tube. The extract was rapidly cooled down and filtered by centrifugal force in a Vac-Elut tube (Analytichem, U.S.A.). The filtrate was dried in a desiccator over NaOH and in vacuum. The residue was redissolved in an appropriate volume of methanol/water (1/1, v/v), and 20 μl of this preparation was used for HPLC analysis. The HPLC equipment consisted of: a high-pressure pump and a four-channel electromagnetic mixing valve, programmable for flow-rate, times, and composition of the eluting solvent (Series 4 HPLC pump, Perkin-Elmer, USA); a column with renewable cartridges of 4.6 � 100 mm Spherisorb ODS2, 3 μm (RSL, Eke, Belgium); a photodiode array detector (model 990, Waters, USA); and a system for data storage, manipulation, and retrieval (NEC APCIII computer with 20 Mb hard disk). Three solvents were used: (A) water, (B) methanol, and (C) 5% (w/v) phosphoric acid in water. The elution program was: 66A/24B/10C: 2 minutes; linear gradient to 0A/90B/10C: 27 minutes; 0A/90B/10C: 3 minutes; flow rate: 1.2 ml/minute, creating a system back-pressure of 17 to 24 MPa. The temperature in the chromatography laboratory was stabilized between 20�C and 22�C. The detection wavelength was selected according to the chemical nature of the peaks present. In general, animal dyes were best analyzed at 275 nm, whereas 255 nm was the optimal detection wavelength for vegetal mordant dyes and 288 nm for indigoids. A test for indigo was performed using the method of reductive bleaching in alkaline medium (Na2S2O5/OH−) that allows reversible reoxidation to indigotin in the air (Hofenk-DeGraaff 1974). Whether or not indigotin was suspected to be present on a yarn, the usual HPLC procedure for mordant dyes was always applied. In some cases, both indigotin and indirubin were detected in this manner. Recently, a whole new chromatographic system was developedfor the separation and characterization of blue arid purple indigoid dyes (Wouters and Verhecken 1991). Unfortunately, this technique was not yet available for the analysis of the early Peruvian yarns. Any dye component was identified according to two criteria: the retention time and the UV-VIS spectrum. The following commercially available products were used as reference products: alizarin, apigenin, carminic acid, ellagic acid, fisetin, indigotin, kampferol, luteolin, morin, purpurin, quercetin, rhamnetin. Pseudopupurin, rubiadin,and xanthopurpurin were given by R. H. Thompson (University of Aberdeen, Scotland). The characterization of dcII, dcIV, dcVII, flavokemesic acid, and kermesic acid, is described by Wouters and Verhecken (1989a, 1989b). Munjistin was identified according to its predominant presence in Rubia munjista and to spectral data in Thomson (1971). Indirubin was synthesized at our Iaboratory by Verhecken. 4 RESULTS4.1 REFERENCE SAMPLESAll analytical data are presented in table 2. The analyses of Dactylopius coccus insects from various origins and of other scale insect dyes was described in detail in previous papers (Wouters and Verhecken 1989a, 1989b). All compositions of cochineal dyeings from Zumb�hl (1979) conform to the data in Wouters and Verhecken (1989a). A dyeing with either Relbunium ciliatum or Galium (species not specified) is represented by one single sample in Zumb�h. According to the predominance of alizarin, Galium should be the source. Roots from Relbunium hypocarpium were obtained from the Royal Botanical Garden (Meise, Belgium). The dyeing of alum-mordanted wool was carried out by the procedure described by Zumb�hl, but the ratio roots/wool was 4/1 instead of 1/1. Both Relbunium dyes contain mainly purpurin, minor amounts of munjistin, and traces of other anthraquinoid products, among which rubiadin could be identified in Relbunium ciliatum. TABLE 2 CHEMICAL COMPOSITION OF REFERENCE DYEINGS The dye from Alnus jorullensis contained important amounts of luteolin, quercetin, and morin, making it rather unusual in the family of the yellow flavonoid dyes. The same is true for Salvia sagitata, which shows high amounts of apigenin and luteolin. Baccaris genistelloides contains three luteolin-like products: lu4, lu6, and lu8, each more abundant than luteolin. The most complex composition was found with Bidens andicola, in which, apart from luteolin as the main component, apigenin, lu1, lu2, luS, fisetin, and an unknown auronic component also occurred. Thus completely different luteolin-like compounds were present in either Baccaris genistelloides or Bidens andicola. Plant species that are supposed to contain tannin material have ellagic acid as the main component, accompanied by traces of quercetin and kampferol. These analytical features are unlikely to form a basis for species determinations with the aid of dye compositions. The Indigofera anil sample was analyzed in a different way. First, a pyridin extract (20 minutes at 100�C; (a) in table 2) was chromatographed according to Wouters and Verhecken (n.d.). Second, the usual acid hydrolysis procedure for mordant dyes was applied 4.2 UNKNOWN SAMPLESFrom 42 textile pieces, 157 samples were taken. Thirty turned out to be naturally colored fiber, and 11 remained unidentified. A first survey of the analytical results evidenced the predominant presence of well-known compounds, which formed the basis for a classification into several main groups. A more detailed consideration of the analytical results for each group enabled the creation of several sub-groups 4.2.1 Main group cochineal [COCH]Carminic acid was found in 46 samples. A typical cochineal analysis is presented in figure 1.
In 16 samples, carminic acid was the only compound that could be characterized. In 13 other samples, carminic acid was accompanied by one or more of the following products: dcII, dcIV, dcVII, flavokermesic acid, and kermesic acid. The relative ratios of dcII, carminic acid, flavokermesic acid, and kermesic acid conform to the data in Wouters and Verhecken (1989a) and to the composition of the reference samples. All 29 dyes are therefore considered to be pure cochineal, prepared from the scale insect Dactylopius coccus, and are designated [COCH]. In 2 samples, carminic acid was present together with purpurin; according to the composition of appropriate reference samples, this mixture represents a combination of cochineal with a vegetal dye from Relbunium. These dyes form the subgroup [COCH-RELB]. The carminic acid/purpurin ratio was totally different in these two samples (1/9 in the first, 7/3 in the second). This variation means that a totally different ratio of insect and plant extracts was originally used for dyeing. However, the actual hue is brown in both cases. In 15 samples, carminic acid was found in combination with indigotin (identified in a separate experiment by reductive bleaching). Occasionally, indigotin was also detected in the acid hydrolysate of these samples; in only two cases indirubin was found as well, in concentrations of up to and for not more than 7% (at 288 nm) relative to indigotin. Any combination of carminic acid with a blue indigoid dyestuff, whatever its composition, is designated [COCH]+[INDI]. 4.2.2 Main group Relbunium [RELB]Purpurin was present in 20 samples. In 2 of them it was the only component detected. In another 7 samples the minor products found were munjistin (≤ 7% relative to purpurin) and xanthopurpurin (≤ 2% relative to purpurin). A representative analysis of this main group is presented in figure 2. Purpurin, pure or in combination with small amounts of munjistin (table 2), surely represents a species from the Relbunium genus and is therefore represented by [RELB].
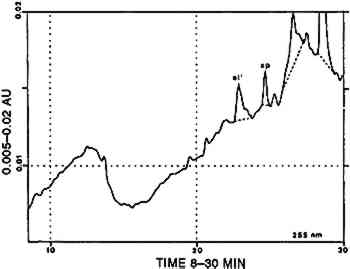
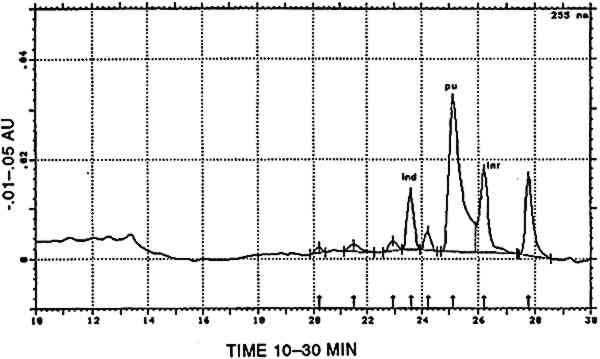
In one sample, munjistin was the most prominent component besides traces of purpurin Combinations with cochineal are referred to as [COCH-RELB] (see sec. 4.2.1) and those with yellow dyes containing luteolin as [LUTE-RELE] (see sec. 4.2.4). Indigotin was also found in five samples (identified by reductive bleaching), all showing both indigotin and indirubin in the acid hydrolysates. In all these samples, indirubin prevailed over indigotin. Any combination of purpurin with blue indigoid dyes is referred to as [RELB]+[INDI]. 4.2.3 Main Group Xanthopurpurin [XAPU]The anthraquinone xanthopurpurin (or purpuroxanthin) is reported to be present in the roots of Rubia tinctorum, R. cordifolia, R. sikkimensis, several Galium species, Asperula odorata, and Morinda umbellata(Thomson 1971). None of these plants occurs in the South American biosphere. Relbunium species, which do occur there, were not reported to contain xanthopurpurin, but mostly purpurin, munjistin, and pseudopurpurin (Fester and Lexow 1943b; Schweppe 1986; Thomson 1971; table 2). Four samples evidenced the presence of xanthopurpurin, with no trace of purpurin but accompanied by an anthraquinone-like dye, spectrally resembling alizarin (fig. 3) and in relative ratios ranging from 67/33 to 80/20 (alizarin-like/xanthopurpurin). A typical chromatogram is presented in figure 4. Several possible interpretations may be given to this phenomenon: Xanthopurpurin may be formed from purpurin by reduction (Sch�tzenberger 1865); however, aging processes mostly involve oxidation reactions, and the same should have occurred with other similar samples that still show purpurin as the most prominent compound (see main group [RELB]). The composition of [XAPU] may also refer to a specific Relbunium species not previously analyzed. Finally, a plant not belonging to the Relbunium genus might be involved. Further research on the plants themselves will be necessary to solve this problem, but the present analytical data as such seem interesting, especially when the historical and geographical context related to [XAPU] is considered (table 6).
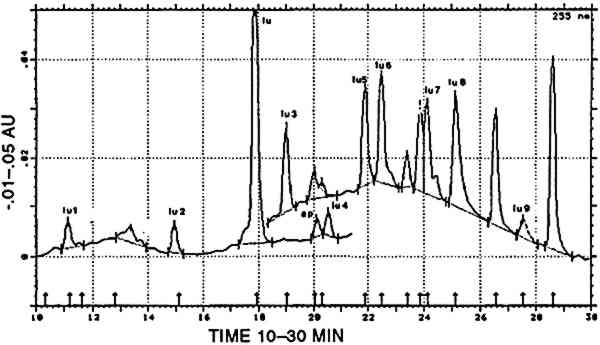
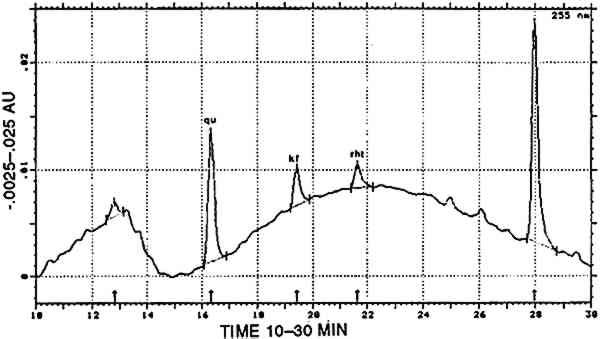
TABLE 6 CORRELATION BETWEEN CULTURAL AND HISTORICAL DATA AND DYESTUFF GROUPS Three [XAPU] samples showed a combination with indigotin (identified by reductive bleaching). Only one of them showed appreciable amounts of indirubin in the appropriate acid hydrolysate. All three are designated as [XAPU]+[INDI]. 4.2.4 Main group luteolin [LUTE]Twenty-six textile samples contained luteolin and/or a series of products that spectrally closely resemble luteolin. Some of these so-called luteolin-like products were also found on modern wool samples dyed with actual Peruvian dye plants (Zumb�hl 1979; table 2). All data concerning these luteolin-like products are given in table 4. Although the differences in the relative retention times of some of these compounds are sometimes very small, different products are indeed involved, since lu3, lu5, lu6, lu7, lu8, and lu9 occur as distinct peaks in the same analysis. Figure 5 is a combination of two chromatograms made to visualize the relative position of each lu-like component. UV-VIS spectra of all these products are given in figure 6. TABLE 4 OCCURRENCE AND CHARACTERIZATION OF LUTEOLIN-LIKE COMPONENTS PRESENT IN THE DYES OF [LUTE] Just two analyses showed luteolin only, due to the small dye recovery that made accompanying products undetectable. Eight samples consisted of luteolin and lu1, with no further addition; in seven samples the relative ratios ranged from 13/87 to 23/77 (lu1/lu), calculated at 255 nm. This very reproducible relative ratio Another subgroup of three samples is characterized by the presence of 5–15% fisetin together with luteolin (always the main component) and lu1: [LUTE-FISE]. Fisetin is known to be a component of the dye prepared from Cotinus coggygria, but its main yellow compound is the accompanying 3′,4′,6-trihydroxyaurone (Wouters and Florquin 1987). However, this aurone never appears in the Peruvian samples, although similar products definitely occur in dyeings with Bidens andicola(table 2). It would not be surprising, however, that aurones remain undetected in historical samples, since 3′,4′,6-trihydroxyaurone was proven to be the less lightfast component in Cotinus coggygria(Wouters and Florquin 1987). The plant source, responsible for the combination of luteolin and fisetin on yarns, was used twice with a vegetal source of the Relbunium type. Thus, a further subgroup [LUTE-RELB] is characterized by the presence of luteolin, fisetin, and purpurin (two samples), luteolin and purpurin each being the main component once. In four samples a combination of luteolin with apigenin was found in a ratio ranging from 95/5 to 87/13 (luteolin/apigenin), which is in very close agreement with the situation in European weld Reseda luteola(Wouters 1987). However, in the Peruvian samples, lu1, lu2, lu3, lu4, and lu7 also occurred in different combinations. This subgroup is called [LUTE-APIG]. A remarkable subgroup, [LUTX], was found to contain numerous luteolin-like products, but no luteolin, lu1, or lu2 is present (seven samples). Instead, lu3, lu5, lu7, and lu8 may each be the main component; lu6 and lu9 were also detected. Any combination of a dye from the [LUTE) group with a blue indigoid dye (identified by reductive bleaching) is referred to as [luteolin subgroup]+[INDI]. It may be emphasized that a combination with indigotin occurred only once in [LUT1], once in [LUT4], never in [LUTE-FISE] and always in [LUTX]. In this last subgroup, in six samples, comparable amounts of indigotin and indirubin were found in the appropriate acid hydrolysates. 4.2.5 Main group tannins [TANN]Two analyses show the presence of ellagic acid, derived from hydrolysable tannins by the acid hydrolysis recovery procedure. Some other compounds with spectral characteristics similar to those of ellagic acid were also found in minor quantities. 4.2.6 Main group quercetin [QUER]This flavonoid is known to be the main coloring principle of Quercus tinctoria, indigenous to North America. It occurs in the wood as the glucoside quercitrin, which is readily converted to quercetin by acid hydrolysis. Apart from this valuable dye source, quercetin also occurs in numerous plants, mostly accompanying other flavonoid dyes. Quercetin was found in four Peruvian samples, once combined with luteolin, once with kampferol and rhamnetin (fig.7). The combination of luteolin and quercetin is rare and occurs also in the modern dyeing with Alnus jorullensis(Zumb�hl 1979; table 2).
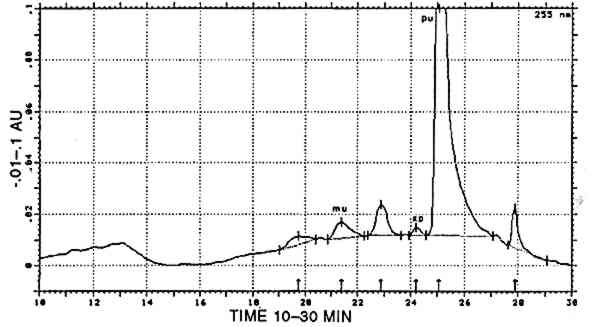
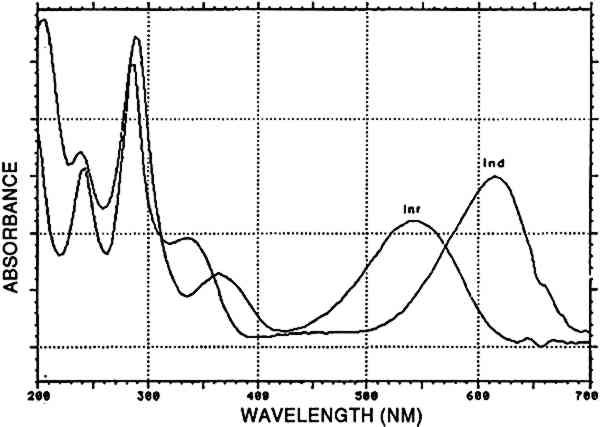
4.2.7 Main group indigotin [INDI]Indigotin detected by reductive bleaching (Hofenk-De Graaff 1974) as well as by HPLC of an acid hydrolysate is represented by the dye group [INDI]. The detection of indigotin and indirubin (fig. 8) in mixtures prepared to solubilize mordant dyes is probably due to the state of deterioration of the dyed fiber. When very deteriorated, the fiber disintegrates in the acid hydrolysis medium, and small indigoid particles may suspend in the liquid. Obviously, the higher solubility of indirubin in the acid hydrolysis medium may considerably alter the ratio between indigotin and indirubin (table 2). In the early Peruvian samples, indirubin was often more abundant than indigotin. The presence of varying amounts of indirubin in commercial indigo preparations was described by Popplewell Bloxam and Perkin (1910). High indirubin amounts may be obtained by manufacturing processes that favor the oxidation of indoxyl to isatin; hence, this feature may indicate particular methods of indigo dye preparation. An example of an analysis showing purpurin, indigotin, and indirubin is given in figure 9.
4.3 COMPILATION OF ANALYTICAL DATAIn table 5 are listed all the fabrics with their code numbers according to museum collection, hue of samples taken from them as judged by the naked eye, and type of dye groups found. Unknowns and naturally colored fibers were omitted. TABLE 5 COMPILATION OF ANALYTICAL DATA WITH REFERENCE TO EACH TEXTILE SAMPLE AND TO THE COLLECTION NUMBER OF EACH FABRIC 5 DISCUSSIONIn this paper modern dyeings with known Peruvian dye sources and dyeings from pre-Columbian Peruvian textiles were analyzed for the presence and relative abundance of dye components. From this study it follows that many more sources than hitherto described may be identified with the aid of present-day dyeings or classified according to the presence of specific compounds. A series of 157 analyses must not be considered large enough to draw conclusions that are statistically tolerable, since the historical, geographical, and cultural delimitations involved are very large. It is therefore preferable to describe the types of dye found in each cultural period (table 6) and to combine these data with previously published results. [RELB] was detected in samples belonging to the Paracas necropolis, Nazca, Huari, and the Tiahuanaco coastal cultures. However, only in the Paracas necropolis and in Nazca was pure [RELB] found, eventually combined with Earlier analytical results from South Peruvian textiles of 100–200 A.D. showed a combination of purpurin and xanthopurpurin (Schweppe 1986) that conforms to our [RELB] from the same period. Cochineal was found in Nazca textiles in an earlier investigation (Fester and Lexow 1943a); however, the presence of insect reds in the Paracas necropolis, as claimed by the same authors (Fester and Lexow 1934), was later contradicted (Fester and Lexow 1943a). The detection of cochineal in Nazca textiles was also mentioned by Saltzman (1978), making reference to Saltzman et al. (1963) where no precise historical data were given. The detection of cochineal in only 3 out of 141 Paracas textiles (Saltzman 1986) may be confirmed by the absence of this dye in our more limited number of samples. It is unlikely that such a low frequency of cochineal detection in the Paracas necropolis, or even in Nazca, would reflect a solid dyeing practice. Indeed, the limited use of a dyestuff so much easier to obtain and with so much higher a yield, as compared to the vegetal one, is without economical or logical sense. The ease and the high recovery with which the animal dye could be obtained certainly may have been responsible for the disappearance of the vegetal dye from Relbunium in the southern coastal regions from the Chancay period on. Another interesting feature of the red dye analyses is the characterization of the [XAPU] subgroup, apparently only encountered in the Paracas necropolis. Moreover, it was never found to occur together with [RELB] on yarns of the same fabric. Probably, [XAPU] represents a dye from a specific plant, used in a historically restricted way. This kind of dye has not been previously described in the context of early Peruvian dyeing practices. Most yellow dye groups do not seem to be specific for any given period: e.g., [LUT1] occurs in the Paracas necropolis, from the Nazca and Chancay periods. [QUER] clearly represents a more recent dye, used from the Chancay to the Inca period. The limited number of reference materials analyzed for this study and the absence of any other data on yellow dyes in the literature make it difficult to assign specific plants to the dye groups found. However, one sample from [QUER] is very possibly from Alnus jorullensis, and [LUTE-FISE] or [LUTE-APIG] might indicate the presence of Bidens andicola, mixed with another yellow dye source (e.g., Baccaris genistelloides). An unusual yellow dye group was named [LUTX] (see section 4.2.4). It was found in textiles from the Paracas necropolis only and, moreover, only in those that also contain [XAPU]. Thus both [XAPU] and [LUTX] might be related to the Paracas necropolis or even to a specific phase of this civilization. More elaborate studies on yellow dyes will have to be carried out to confirm this hypothesis. The detection of several hydroxyflavone dyes on Peruvian textiles dated 100–200 A.D., without characterization of the components (Schweppe 1986), is likely to refer to [LUTX], since at least luteolin would have been recognized by this author. The use of tannins, represented by [TANN], was both rare and historically unspecific; they appear once in the Paracas necropolis and once in the Inca period. The use of indigoid dyes may be regarded as universal, both to dye blue and in combination with any mordant dye. More study will be 6 CONCLUSIONSDye analyses of pre-Columbian Peruvian textiles were classified according to the most characteristic chemical dye component present ([XAPU], [LUTE], [QUER], or [INDI]) or according to a familiar name for the dye or the dye source involved ([RELB], [COCH], or [TANN]). In some cases subgroups were created that allowed a more refined classification of related analytical patterns: e.g., [LUTX] in [LUTE]. The dyes represented by [XAPU] and [LUTX] were found only in the Paracas necropolis. [RELB] was also found in the Nazca period. During the Huari and Tiahuanaco cultures (500–1100 A.D.), [COCH] was found together with vegetal dyes [PURP]. From the Chancay period on, [COCH] was the only red dye detected. In this paper, [COCH] was not found in Paracas or Nazca, but it was found during those periods in other studies. An additional series of analyses, in combination with radiocarbon dating by accelerated mass spectrometry (Van Strydonck n.d.), may improve our knowledge about the historically and regionally dependent use of cochineal. The HPLC method used here seems to be a workable tool not only for the recognition of red dyes but for the yellows that were previously considered of less informative value (Saltzman 1986) and for indigoid dyes, pure or combined with mordant ones. A limited study of actual yellow-producing Peruvian dye plants already indicates that a great variety in composition exists and that exactly these variations may be useful in further studies involving HPLC to assign specific plant species to early Peruvian dyeings. ACKNOWLEDGEMENTSThe association of N. Rosario-Chirinos to the laboratory of the Royal Institute of Cultural Heritage, Brussels, was possible through a credit given by the Ministry of Foreign Affairs, Brussels. A sample of Relbunium hypocarpium root was provided by the Royal Botanical Garden, Meise, Belgium. A sample of Relbunium ciliatum–dyed yarn was kindly given by Helmut Schweppe. A collection of yarns dyed with actual Peruvian dye plants (Zumb�hl 1979) was provided by Max Saltzman, Institute of Geophysics and Planetary Physics, University of California, Los Angels. Samples of pseudopurpurin, rubiadin and xanthopurpurin were given by R.H. Thomson, University of Aberdeen, Scotland. REFERENCESAntunez de Mayolo, K. K.1989. Peruvian natural dye plants. Economic Botany43:181–91. Baizerman, S.1985. Introduction to pre-Columbian double cloth. In Double-woven treasures from old Peru, ed.A.Cahlander. St. Paul, Minn.: Dos Tejedoras Publishing. 3–6. Born, W.1938. Scarlet. Ciba Review7:206–27. Brunello, F.1973. The art of dyeing in the history of mankind. Vicenza, Italy: N. Pozza. Donkin, R. A.1977. Spanish red: An ethnographical study of cochineal and the Opuntia cactus. Transactions of the American Philosophical Society67:1–84. Fester, G. A.1940. Los colorantes del antiguo Peru. Archeion22:229–41. Fester, G. A., and J.Cruellas. 1934. Colorantes de Paracas. Revista del Museo Nacional de Lima3:5 pp. Fester, G. A., and S. G.Lexow. 1943a. Las raices del g�nro Relbunium en la tintorer�a Americana. Anales de la Sociedad Cient�fica Argentina136: 233–40. Fester, G. A., and S. G.Lexow. 1943b. Colorantes de insectos. Anales de la Sociedad Cientif�ca Argentina135:89–96. Geiss-Mooney, M. E., and H. L.Needles. 1981. Dye analysis of a group of late intermediate period textiles from Ica, Peru. In Preservation of paper and textiles of historic and artistic value II, ed.J. C.Williams. Advances in Chemistry Series no. 193. Washington, D.C.: American Chemical Society. 291–300. Gibaja Oviedo, S., and L.Salazar de Cavero. 1977. Producci�n de la p�rpura de Tiro a partir del molusco concholepas Chanque. Bolet�n de la Sociedad Qu�mica del Peru43(13):139–40. Haslam, E.1966. Chemistry of vegetable tannins. New York: Academic Press. Herrera, F. L.1939. Estudios sobre la flora del Departemento del Cuzco, vol. 1. Hofenk-De Graaff, J. H.1969. Natural dyestuffs, origin, chemical constitution, identification. ICOM Committee for Conservation Preprints, 2d Plenary Meeting, Amsterdam. 69/16. Hofenk-De Graaff, J. H.1974. A simple method for the identification of indigo. Studies in Conservation19:54–55. Kandt, V. B.1979. Peruaanse textielen.Rotterdam: Museum voor Landen Volkenkunde. Kuramoto, N., and T.Kitao. 1979. Contribution of singlet oxygen to the photofading of indigo. Journal of the Society of Dyers and Colourists95(9):257–61. Martoglio, P. A., S. P.Bouffard, A. J.Sommer, and J. E.Katon. 1990. Unlocking the secrets of the past.: The analysis of archaeological textiles and dyes. Analytical Chemistry62:1123A–28A. Popplewell Bloxam, W., and A. G.Perkin, 1910. Indirubin, Part 1. Journal of the Chemical Society97:1460–75.
Saltzman, M.1978. The identification of dyes in archaeological and ethnographic textiles. In Archaeological chemistry II, ed.G. F.Carter. Advances in Chemistry Series no. 171. Washington D.C.: American Chemical Society. 172–85. Saltzman, M.1986. Analysis of dyes in museum textiles or, you can't tell a dye by its color. In textile conservation symposium in honor of Pat Reeves, ed.C. C.McLean and P.Connell. Los Angeles: Los Angeles County Museum of Art. 27–39. Saltzman, M., A. M.Keay, and J.Christensen. 1963. The identification of colorants in ancient textiles. Dyestuffs44(8):241–51. Sch�tzenberger, M. P.1865. Recherches sur les mati�res colorantes de la garance. Bulletin de la Soci�t� Chimique de Paris4:12–17. Schweppe, H.1986. Identification of dyes in historic textile materials. In Historic textile and paper materials: Conservation and characterization, ed.H. L.Needles and S. H.Zeronian. Advances in Chemistry Series no. 212. Washington, D.C.: American Chemical Society. 153–74. Thomson, R. H.1971. Naturally occurring quinones. 2d ed.New York: Academic Press. Van Strydonck, M. n. d. Datering van pre-Columbiaanse textilen. Unpublished manuscript. Verhecken, A., and J.Wouters. 1988–89. The coccid insect dyes. Historical, geographical, and technical data. Bulletin of the KIK/IRPA22:207–39. Wouters, J., 1985. High performance liquid chromatography of anthraquinones: Analysis of plant and insect extracts and dyed textiles. Studies in Conservation30:119–28. Wouters, J.1987. Analyse des colorants des tappisseries brugeoises des XVIe et XVIIe si�cles. In Bruges et la tapisserie. Brugges, Mouscron, Belgium: 515–26. Wouters, J., and S.Florquin. 1987. The colouring matter of Rhus cotinus.Dyes on Historical and Archaeological Textiles6:28–31. Wouters, J., and A.Verhecken. 1989a. The coccid insect dyes: HPLC and computerized diode array analysis of dyed yarns. Studies in Conservation34:189–200. Wouters, J., and A.Verhecken. 1989b. The coccid insect dyes: Species recognition by HPLC and diode array analysis of the dyestuffs. Annales de la Soci�t� Entomologique de France25(4):393–410. Wouters, J., and A.Verhecken. 1991. HPLC of blue and purple indigoid natural dyes. Journal of the Society of Dyers and Colourists. 7:266–69 Yacovleff, E., and F. C.Muelle. 1934. Notas al trabajo: Colorantes de Paracas. Revista del Museo Nacional de Lima3:154–68. Zumb�hl, H.1979. Tintes naturales. Huancayo, Peru: Kamaq Maki. AUTHOR INFORMATIONJAN WOUTERS earned a Ph.D. in chemistry with a specialty in biochemistry form the State University of Gent, Belgium, in 1978. Since 1982, he has been involved in the chemical analysis of natural organic materials at the Koninklijk Instituut voor het Kunstpatrimonium. Address: Jubelpark 1, B-1040 Brussels, Belgium. NOEMI ROSARIO-CHIRINOS has a degree in chemistry from the Universidad Nacional Mayor de San Marcos, Lima, Peru, and has done post-graduate work in conservation science at New York University; Instituto Nacional de Cultura, Direccion de Conservacion del Museo Nacional, Av. Javier Prado y Aviacion–San Borja, Lima Peru.
 Section Index Section Index |