CURRENT RESEARCH ON THE EFFECTS OF SOLVENTS AND GELLED AND AQUEOUS CLEANING SYSTEMS ON OIL PAINT FILMSJIA-SUN TSANG, & DAVID ERHARDT
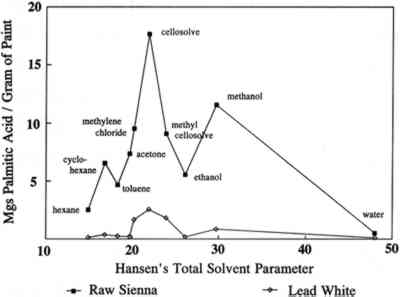
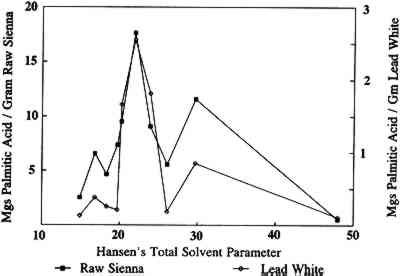
ABSTRACT—Some current research, in the authors' laboratories and elsewhere, on the effects of solvent and aqueous-based cleaning systems is reviewed. The application of varnish is considered in terms of the exposure of the paint film to the solvents used to dissolve and apply the varnish. The varnish and solvent solution is seen as able to act as a poultice in leaching soluble components from the paint film. The action and effects of some aqueous and gelled systems also are considered. Initial data indicate that soaps and gels behave in some ways much as solvents do, both in the time frame of their action and the amounts of materials that they can remove from paint films to which they are applied. 1 INTRODUCTIONIt has long been known that solvents may remove portions of the oil media from dried paint films. This phenomenon was studied beginning around 1950 by both Graham and Stolow (Graham 1953; Feller et al. 1985). At the time, the choice of cleaning agents for paintings primarily consisted of either pure or mixed solvents. Conservators had to choose a solvent system that removed layers of dirt, varnish, and overpaint while affecting the original paint layer as little as possible. This decision often involved a choice between solvents with short cleaning times and fast penetration versus longer cleaning times and slow penetration. In the last few years, the range of choices has been expanded to include gelled solvents and aqueous cleaning systems (Wolbers et al. 1990). Gelled systems are used on the assumption that penetration into the original paint layer will be retarded while only minimally reducing the strength of the reagents, thus providing the conservator with more control of the cleaning process. Soaps are prepared from compounds that are chemically similar to the materials they are supposed to remove; this process is intended to produce a soap that removes these materials preferentially. 2 SOLVENTS AND SOLVENT PARAMETERSInitial studies carried out at the Conservation Analytical Laboratory, Smithsonian Institution, on the types and amounts of materials leached from oil paint films during soaking in various solvents have produced results that confirm Stolow's work and demonstrate the effects of pigments and age (Erhardt and Tsang 1990). Free fatty acids and their degradation products were found in the extracts, and the amount varied with the solvent, pigment, age of the film, and nature and length of solvent exposure. Materials other than oil components were also extracted from some samples. For example, wax and synthetic dyes were found in some extracts. Such additives often are found in paints, especially modern ones. Waxes can be used as extenders or may be added to pigments to aid in mechanical processing. Dyes may be used as extenders for inorganic pigments or may replace them entirely. The presence of such Lead white pigmented oil films were found in general to be less affected by solvents than films with other pigments that were studied (Erhardt and Tsang 1990). This is not surprising, since lead white speeds up the cross-linking of drying oils and may also form insoluble salts with the free fatty acids. Such pigment-medium bonding should reduce the sensitivity of the paint film to solvents. Raw sienna films, on the other hand, lost up to one-third of their weight after soaking in acetone and ethanol; most of this loss was due to the loss of pigment. This loss indicates a pigment-medium bond that is either weak or easily disrupted. We have looked at the amounts of material extracted from oil paint films by various solvents during varying types and lengths of exposure. The amounts of free palmitic acid extracted by various solvents over six hours versus the Hansen's total solvent parameter for each solvent are plotted in figure 1. Because of the limited size and number of aged samples available, only one measurement was conducted for each point. In a few cases where we have reported experiments, the results have varied within a range of about � 10%. The reliability of the data is further supported by the way in which trends in the data are maintained over time, through ranges of solvents, and for different types of samples. Hansen's parameters are measures of the contributions of three factors (dispersion forces, dipole interactions, and hydrogen bonding) to solvent interactions (Barton 1983). Hansen's total solvent parameter is a vector sum of these contributions and is a measure of the total solvent strength. Even though the amounts extracted from raw sienna films are consistently larger than those from lead white films, the shapes of the curves are very similar. This similarity can be seen more readily in figure 2, where the scale for the lead white plot is expanded. The similar shape of these two curves indicates that the lead white and raw sienna paint films differ only in the degree of their sensitivity to individual solvents, not in regard to which solvents they are sensitive. Thus, the effect of lead white pigments on paint films is to reduce the overall sensitivity of the film to solvents, not to shift this sensitivity to more or less polar solvents. This finding would indicate that the reduced sensitivity to solvents of lead white films is due to an increase in cross-linking, the formation of insoluble salts, or some other mechanism that has little effect on the polarity of the paint film. For instance, a large increase in oxidation of the paint film catalyzed
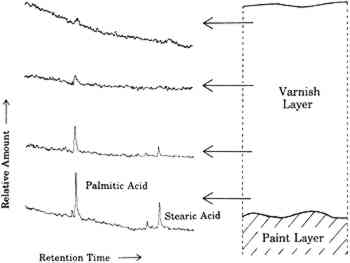
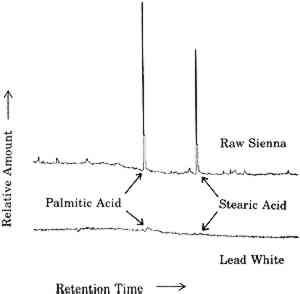
The sensitivity toward solvents of these paint films is not a smooth function of Hansen's total solvent parameter, however, as demonstrated by the two dips about the central peak. That this plot is not a smooth curve is the result of trying to plot sensitivity to solvents, which is a function of at least three distinct properties of the solvents, against a single parameter. Similar anomalies are encountered even in a standard Teas plot, which is an attempt to compress a plot of a function of three variables into two dimensions (Teas 1968). Michalski dealt with this problem by “decompressing” the Teas plot in order to make better sense of experimental results (Michalski 1990). Even clearer plots eventually may be obtained by abandoning the Teas plot format altogether and directly plotting results in three dimensions as a function of the three Hansen's parameters. 3 VARNISHES AS POULTICESPaintings are exposed to solvents at times other than during cleaning. One of the more prolonged exposures of a painting to solvents is during the application and drying of varnish solutions. Solvent evaporates from the surface of the varnish solution, but some also diffuses into the paint film. Solvent that diffuses into the paint film may dissolve soluble components. As this solvent eventually diffuses back into and through the varnish layer and evaporates from the surface, it may carry the soluble oil components with it. These involatile compounds may be left behind either in or on the varnish layer. This phenomenon is demonstrated by the following example. A layer of natural resin varnish was scraped from a small area of an oil painting in a series of ten steps. These ten scrapings were analyzed by gas chromatography according to previously reported methods to look for differences among samples from different levels in the varnish layer (Erhardt et al. 1988). Portions of the gas chomatograms where free fatty acids (palmitic and stearic) would appear are shown for four of these levels in figure 3. There are only traces of fatty acids in the top of the varnish layer. Larger amounts of fatty acids are seen in levels closer to the paint surface. A poulticing action of the applied varnish in moving these fatty acids from the paint layer to the varnish is not the only possible explanation, however. It is possible that fatty acids simply diffused into the paint layer over time or that fatty acids were present in the original varnish (which was present on the painting when acquired). To rule out these alternative explanations, a solution of Acryloid B72 was applied in xylene solution to five-year-old raw sienna and lead white oil paint films. Acryloid B72, manufactured by Rohm and Haas, Philadelphia, Pennsylvania, is a synthetic acrylic polymer commonly used as a varnish. It does not contain any fatty acids. After several days of drying, the B72 was peeled off and dissolved in methylene chloride. This solution was extracted with a solution of sodium bicarbonate, which was then separated, acidified, and extracted with fresh methylene chloride.
The fact that fatty acids are extracted from paint films by the application of varnishes is not necessarily cause for alarm. Any treatment of an oil paint film that involves the use of solvents will remove some material from the paint, even if in amounts detectable only by very sensitive analytical techniques. The presence of fatty acids in any amount does, however, demonstrate that an applied layer of varnish solution can act as a poultice, with the solvent drawing soluble materials from the paint film up into the layer of varnish. One does not apply just a varnish, one applies a varnish and a solvent. These results emphasize that the choice of solvents used in the application of a varnish to a painting is just as important as the choice of solvents used to clean the painting. Such considerations provide added importance to the efforts to find or develop varnishes that are and remain soluble in the mildest solvents possible (de la Rie and McGlinchey 1990). One important consideration of the poulticing effect of the application of solutions of varnish is in the interpretation of analytical results. Usually, the presence of fatty acids and terpenoid components in a single layer has been thought to indicate the use of an oil-resin mixture. Such mixtures, containing various proportions of oil and resin, have been used as glazes and varnishes. This work shows that fatty acids may be present in a resin layer due to extraction from the underlying paint layer rather than to the addition of oil to the resin. A modification of analytical techniques may be required to determine the source of the fatty acids. If a varnish is dissolved in an organic solvent and this solution is extracted with a weakly alkaline aqueous solution, any free fatty acids poulticed from the paint film would be extracted into the aqueous layer. If oil were a component of the varnish, there will be fatty acids present as glyceride esters. These esters will remain in the organic layer and could be detected by subsequent hydrolysis, methylation, and gas chromatographic analysis of fatty acids. 4 WATER AND AQUEOUS CLEANING SYSTEMSAlthough there exists a large body of work on the effects of solvents on oil paint films, more work is necessary. However, there is even less information available on the effects of aqueous cleaning systems on oil films. Some work on
One class of aqueous cleaning system in current use in the treatment of paintings includes gelled soap formulations. Richard Wolbers, who pioneered the use of these systems, has used them to approach the problem of the removal of varnish from the aqueous end of the solvent spectrum. Some considerations would suggest that aged and oxidized terpenoid varnishes should be more soluble in more polar solvents and that mixtures containing soaps formed from resin acids would be especially effective in the removal of resin varnishes. Several papers presented at the 1990 Brussels conference of the International Institute for Conservation of Historic and Artistic Works examined the use of aqueous cleaning systems for the cleaning of paintings. Wolbers (1990) used radioisotopically labeled acids to form soaps and then quantified the residues left when these soaps were applied to paint films. The amounts of residues were found to depend on factors such as pH, soap concentration, and counterion. However, significant amounts of material were found to be left behind with all three detergents studied: deoxycholates, abietates, and palmitates. Burnstock and White (1990) also reported problems with the clearance of soap formulations. They concluded that gelled soap formulations should be tailored to act only on the uppermost layer and that careful attention should be paid to the clearance of the soap. They also recommended against the use of Triton X-100 as a gel component, citing evidence of oxidation of the soap components during its use. Recent investigations by the authors have also included gelled and soap systems. The initial data indicate that these systems remove components of the paint film just as solvents do. It is not surprising that formulations intended to remove varnishes and overpaint would also act on original paint layers. In fact, Koller (1990) reported evidence that soaps based on deoxycholic acid may solubilize oil components in preference to resins. A gel soap solution of triethanolammonium abietate was prepared according to the recipe suggested by Wolbers (Wolbers et al. 1990). The only change was the elimination of surfactants such as Triton X-100 following the recommendations of Burnstock and White. This mixture was applied to samples of 13-year-old raw sienna pigmented linseed oil films. The gel was cleared as suggested using cotton swabs and hexane twice, followed by a final rinse of the paint film with hexane. The gel, swabs, and hexane were saved and combined for the analysis of extracted palmitic acid by gas chromatography. Treatment with gelled ethanol also was studied. The results of the analyses of the extracts from several different treatments are listed in table 1. As in previous work (Erhardt and Tsang 1990), the amount of free palmitic acid extracted from the paint film is used to evaluate the detergent effect of the formulation in removing material from the paint film. TABLE 1 AMOUNT OF FREE PALMITIC ACID EXTRACTED FROM SAMPLES OF A 13-YEAR-OLD RAW SIENNA OIL PAINT FILM As can be seen in the data for gelled and ungelled ethanol, gelling reduces only slightly the amount of palmitic acid extracted in a period of six hours. It can also be seen in the data for the abietate soap solution that reducing the exposure time for the abietate soap from 6 hours to 15 minutes also reduces only slightly 5 CONCLUSIONSWork on the mechanisms and effects of cleaning systems for oil paintings continues. Hansen's solvent parameters provide one way to evaluate trends that appear in the effects of solvents. The Teas plot, which compresses the three variables of solvent properties into a two-dimensional plot, is suitable for general use but may result in some anomalies. The effects of solvents must be considered whenever they are applied to a paint film, even when they are not specifically being used for cleaning. Solvents used to apply varnishes also act on paintings, and the varnish-solvent mixture can act as a poultice in leaching soluble materials from a paint film to which it is applied. There is less information on the process and effects of the use of gelled and soap solutions. It is clear, however, that these materials behave in some ways much like solvents. More study of the use of these materials is required, especially on their mode of action and the possible continuing effects of the residues of the involatile materials that they contain. REFERENCESBarton, A.F.M.1983. CRC handbook of solubility parameters and other cohesion parameters. Boca Raton, Fla: CRC Press, Inc. Burnstock, A., and R.White. 1990. The effects of selected solvents and soaps on a simulated canvas painting. In Cleaning, retouching and coatings: Technology and practice for easel paintings and polychrome sculpture, ed.J. S.Mills and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 111–18. de la Rie, E. R., and C. W.McGlinchey. 1990. New synthetic resins for picture varnishes. In Cleaning, retouching and coatings: Technology and practice for easel paintings and polychrome sculpture, ed.J. S.Mills and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 168–73.
Erhardt, D., W.Hopwood, M.Baker, and D.von Endt. 1988. A systematic approach to the instrumental analysis of natural finishes and binding media. AIC preprints, 16th Annual Meeting, American Institute for Conservation, Washington, D.C. 67–84. Erhardt, D., and J.Tsang. 1990. The extractable components of paint films. In Cleaning, retouching and coatings: Technology and practice for easel paintings and polychrome sculpture, ed.J. S.Mills and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 93–97. Feller, R. L., N.Stolow, and E. H.Jones. 1985. On picture varnishes and their solvents. Rev. and enl. ed.Washington, D.C.: National Gallery of Art. Graham, I.1953. The effect of solvents on linoxyn films. Journal of the Oil and Color Chemists' Association36:500–506. Hedley, G., M.Odlyha, A.Burnstock, J.Tillinghast, and C.Husband. 1990. A study of the mechanism and surface properties of oil paint films treated with organic solvents and water. In Cleaning, retouching and coatings: Technology and practice for easel paintings and polychrome sculpture, ed.J. S.Mills and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 98–105. Koller, J.1990. Cleaning of a nineteenth-century painting with deoxycholate soap: Mechanism and residue studies. In Cleaning, retouching and coatings: Technology and practice for easel paintings and polychrome sculpture, ed.J. S.Mills and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 106–10. Michalski, S.1990. A physical model of the cleaning of oil paint. In Cleaning, retouching and coatings: Technology and practice for easel paintings and polychrome sculpture, ed.J. S.Mills and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 85–92. Teas, J. P.1968. Graphic analysis of resin solubilities. Journal of Paint Technology40(516):19–25. Wolbers, R., N. T.Sterman, and C.Stavroudis. 1990. Notes for workshop on new methods in the cleaning of paintings. Marina del Rey, Calif.: Getty Conservation Institute. Wolbers, R.1990. A radioisotopic assay for the direct measurement of residual cleaning materials on a paint film. In Cleaning, retouching and coatings: Technology and practice for easel paintings and polychrome sculpture, ed.J. S.Mills and P.Smith. London: International Institute for Conservation of Historic and Artistic Works. 119–25. AUTHOR INFORMATIONJIA-SUN TSANG is a 1985 graduate of the University of Delaware/Winterthur Conservation Program. She was with the National Gallery of Art in Washington, D.C., from 1984–87 before joining the Conservation Analytical Laboratory, Smithsonian Institution, in 1988 as paintings conservator. She was a specialist in clinical chemistry at the Medical College of Ohio, Toledo, Ohio, from 1974–80. Address: Conservation Analytical Laboratory, Museum Support Center, Smithsonian Institution, Washington, D.C. 20560. DAVID ERHARDT received his Ph.D. from the University of Maryland in 1977. He was a medical research associate at George Washington University from 1978–79 and is currently senior research chemist at the Conservation Analytical Laboratory of the Smithsonian Institution where he has been since 1979. Address: Conservation Analytical Laboratory, Museum Support Center, Smithsonian Institution, Washington, D.C. 20560.
 Section Index Section Index |