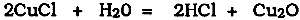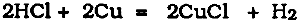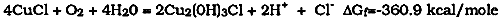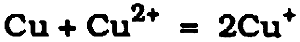BRONZE DISEASE: A REVIEW OF SOME CHEMICAL PROBLEMS AND THE ROLE OF RELATIVE HUMIDITYDAVID A. SCOTT
3 THE REACTIONS OF CUPROUS CHLORIDECuprous chloride is the principal agent of bronze disease. There remains, however, some confusion concerning both the RH values at which cuprous chloride is stable and the reactions that may occur when cuprous chloride in a bronze artifact is exposed to air or becomes unstable. The equations Organ proposed (1963) were the first to be generally accepted as an explanation for the process, but it is now generally recognized that these equations are too simplistic. They suggested that the principal mechanism is the production of cuprous oxide (cuprite) by hydrolysis of cuprous chloride:
The hydrochloric acid generated by this reaction will then produce more cuprous chloride:
The problem with this model was that in isolation the first reaction, that of cuprous chloride with water, will not produce hydrochloric acid and cuprite. As MacLeod (1981) has already pointed out, the ΔG of reaction for equation [4] is +16.3 kcal. mole−1, showing that the reaction will not proceed spontaneously because of the positive value of ΔG. The thermodynamic values used in this discussion refer to the standard free energies of formation of the compounds from the elements and are therefore in the standard state at 25�C. As such they can only be used as a guide to the reactions that will actually occur, for under bronze disease conditions most reactants will not be in their standard states. Nonetheless, they are offered here as a model for further refinement and discussion. In standard conditions, the positive sign of the ΔG of reaction for the hydrolysis of nantokite means that the reaction cannot proceed as such without an additional thermodynamic driving force. In addition, nantokite has a solubility of about 0.006 g per 100 ml of water at room temperature; this low solubility limits the extent to which hydrolysis reactions may occur. It is possible to provide a thermodynamic thrust to the right hand side of equation [4] if the positive ΔG can be overridden by other factors. One of the factors that must be considered is the presence of alloying elements: tin in bronze or zinc in brass for example. If a drop of water is added to cuprous chloride spread over the surface of a piece of brass, gas bubbles evolve from the droplet and a film of cuprite develops on the brass surface. The same reaction on a sample of a high tin bronze (24% tin) is not sufficiently favorable to produce cuprite as an immediate product, even though the ΔG of reaction of tin with hydrochloric acid is −8.856 kcal. mole−1. There are, of course, electrochemical factors and kinetic factors to be considered here. There is obviously an additional driving force for the reaction if carried out on a metal surface lower in the electrochemical series than copper, which is one reason why the reaction with brass is so rapid. Not only are there subtle electrochemical factors to be considered concerning the difference in potential between tin-rich phases and the copper-rich alpha phase in bronzes, but there are a variety of burial conditions to be considered. A simple examination of the likelihood of the tin- or copper-rich phases corroding suggests that tin would be the most likely to corrode. The enthalphy of formation of cuprous oxide or cupric oxide is some 100 kcal. mole−1 less than that for stannic oxide, which should mean that loss of tin is the preferred reaction when the bronze corrodes. But corrosion of the copper-rich phase or the tin-rich phase is mostly dependent on the partial pressure of oxygen, as shown by the work of MacLeod (Taylor and MacLeod 1985). In seawater, MacLeod found that exposure to well-oxygenated conditions resulted in the copper-rich alpha phase being attacked, while in less oxidizing conditions the tin-rich delta phase was attacked. The concentration of tin compounds or copper compounds that might form in the patina will therefore be strongly affected by this selective corrosion phenomenon. What happens in practice when cuprous chloride is in contact with copper and a drop of water is added? In this case there is no cuprite formation; the copper trihydroxychlorides are the principal products. The standard way of making paratacamite, namely the immersion of a sheet of copper in a solution of cupric chloride, produces first a thin layer of cuprite over the copper followed by a layer of paratacamite. Cuprite can be formed as a thin layer adjacent to copper if cuprous chloride and copper are mixed together and regularly moistened with water, but this is not the principal reaction. On copper, cuprous chloride slurries develop a pH of about 3.5–4.0, and the solution develops a green precipitate; one of the copper trihydroxychlorides is formed (Tennent and Antonio 1981). The reaction is one of oxidation and hydrolysis of the cuprous chloride, which takes place with a negative free energy of formation.
This equation has been written using a value for the free energy of formation of paratacamite of −319.8 kcal. mole−1. When cuprous chloride is placed on moist filter paper, the cuprous chloride slowly changes to give mostly atacamite, as reported by Tennent and Antonio (1981). The work of Sharky and Lewin (1971) established that one of the critical factors involved in whether one copper trihydroxychloride isomer or another is formed during possible transformations leading to the formation of atacamite or paratacamite was the concentration of complex cupric chloride ions in solution. Lewin (1973) argued incorrectly that the concentration of the complex copper ions in solution during natural corrosion processes in the soil would be low. With low concentrations, paratacamite is the most favored product, and Lewin posited that the detection of a mixture of paratacamite and atacamite in a patina implied that the corrosion was artificially induced. Lewin's argument suffers from two serious drawbacks: first, the x-ray diffraction data for several natural patinas examined by different laboratories has established that, indeed The corresponding anodic reaction is less well understood. Lucy suggested that the cuprous ions inside the pit are oxidized to cupric ions. This increase in cupric ion concentration disturbs the equilibrium between metallic copper, cuprous, and cupric ions. Copper can then dissolve to maintain equilibrium. In aqueous conditions contiguous with a copper surface the following reactions must be considered:
Lucy proposed that the balance between equations [7], [8], and [9] was responsible for the precipitation of cuprous chloride. If, however, as a result of equation [7], the rate of formation of cuprous ions exceeds conversion into cuprite or cupric compounds, then a layer of cuprous chloride can form. The four essential equations Lucy proposed were
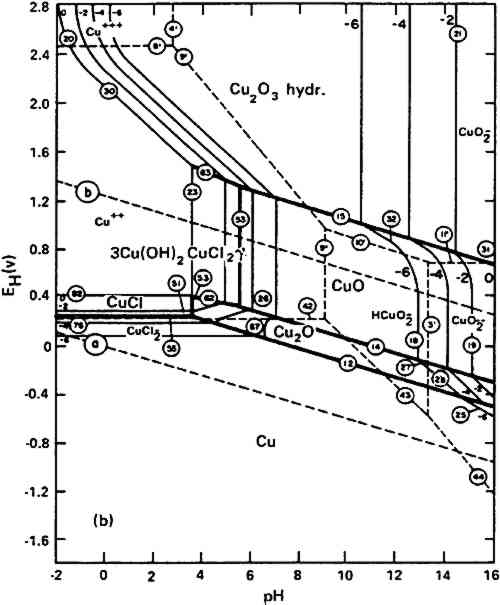
The work of Pourbaix (1976) suggested that similar products were formed in the corrosion pits studied on copper that had been attacked by domestic water. The potential-pH equilibrium diagram for the ternary system Cu-Cl-H2O for solutions containing 10−2 g-ion Cl−/liter, which is about the amount of chloride ion present in a solution saturated in CuCl, is shown in figure 1. In neutral solutions, at pH 7, the diagram suggests that CuCl should hydrolyze according to equation [4], but such neutral conditions are not encountered inside corrosion pits where copper, cuprite, and nantokite coexist.
Examination of the Pourbaix diagram suggests that these three components will be stable at a pH of 3.5 and an E of +270 mVshe. Under these conditions the corrosion reaction is reversible: the pit will grow if the electrode potential inside the pit is higher than +270 mVshe, while the growth will stop and metallic copper can be redeposited if the electrode potential is lower than +270 mVshe. The equilibrium values expected for the pH in corrosion crusts where copper, cuprite, and nantokite are present are therefore acidic and will contain potentially high amounts of complex copper chlorides and therefore act contrary to the model proposed by Sharkey and Lewin (1971). Sharkey and Lewin found that when the CuCl+ concentration reached 20%–30% of the copper ions in solution at a pH of about 4, then atacamite was favored over paratacamite; with still higher copper complexes, such as CuCl2, CuCl3−,CuCl42−, paratacamite once again became the favored species. There is potential for great variation in the concentration of the complex cupric ions in solution, and it is difficult to envisage how the relative amounts of the different isomers can be used to obtain any useful information on burial conditions. The relationship between cuprous chloride and other copper hydroxychlorides, such as calumetite, is less clear. Calumetite—copper hydroxychloride, Cu(OH)Cl—has been identified only a handful of times in the natural patina of ancient bronzes by the work of Nielsen (1977), Meyers (1977), and Helmi and Iskander (1985). Calumetite is at present enigmatic: the conditions under which this mineral can form are not known and, in addition, it is not known whether calumetite may have been formed on the bronzes in question as a result of chemical cleaning treatment. However, since it is known as a mineral and has been found in widely disparate objects it deserves serious attention.
Sharkey and Lewin (1971) found that there was no dimorphic interconversion between paratacamite and atacamite under the conditions they investigated. It has been assumed that the relative proportions of the isomers could afford some clue to provenance or perhaps authenticity of the patinas of different objects. This assumption is not really feasible for the reasons that have been discussed above; the proportions may vary on one object sampled from different locations depending on whether the object has incipient bronze disease and fresh outbreaks of one of the copper trihydroxychlorides have occurred, or the original patina constituents are examined. Even under laboratory conditions the mode of production of the basic chlorides is very critical. If cupric chloride solution is added to calcium carbonate and stirred then atacamite is produced, but if left unstirred then botallackite is formed (Tennent and Antonio 1981). Subtle factors control the conditions under which the different products may form. Some reactions are more repeatable than others. For example, the reaction between cuprous chloride, copper foil, water, and air in an experiment carried out by the author gave mostly paratacamite, in agreement with most of the previously reported results, while the same reaction, replacing cuprous chloride with cupric chloride, gave a mixture of paratacamite and atacamite, with more atacamite. Tennent and Antonio found that the latter reaction invariably produced paratacamite. Unless all the parameters of the reactions, such as pH, temperature, A series of experiments was conducted by Scott and O'Hanlon (1987) using a variety of cuprous chloride powders; some were freshly made in the laboratory and stored under nitrogen, while others were commercial products. The chemicals used in this series of experiments were all analytical grade reagents with very low levels of impurities present (less than 0.1%). The reaction products were sampled after a period of 5 days, and the temperature employed was room temperature (20�C). The experimental work was conducted to determine what the most common products would be when different combinations of the following reactants were employed: copper foil, cuprous chloride, and sodium chloride, cuprous oxide. Analysis was carried out by x-ray powder diffraction using a Debye-Scherrer camera and by Fourier transform infrared spectroscopy. The cuprous chloride powders were prepared by the following methods:
The same series of reactions was also attempted under nitrogen. If oxidation and hydrolysis of cuprous chloride is the principal reaction, then placing the reactants in an inert atmosphere should result in very little or no reaction. This was indeed found to be the case, and in most of the reaction mixtures only slight alteration could be found after analysis by x-ray powder diffraction. The experiments above illustrate the comparative difficulty of synthesis of botallackite under ordinary laboratory conditions, a fact borne out by the analysis of the products of bronze disease on antiquities: botallackite is rarely reported, while paratacamite and atacamite predominate. Some of the experiments were designed to see what products might be expected from the reaction between copper metal and sodium chloride to simulate events that may occur in highly saline environments, as well as to determine the reactions between cuprous chloride and copper foil in the presence of moist air or with added water. It is interesting that the cuprite in experiment 8 is attacked during the reaction with cuprous chloride and the red color of cuprite gradually changes, the whole mass becoming pale green. It is No change was observed in a similar experiment where the cuprous chloride was replaced by sodium chloride. Reaction did ensue between copper powder and sodium chloride with the formation of atacamite. Although more complex reactions may sometimes occur in highly saline environments, leading to complex copper salts, such a reaction was not observed here. As a result of the increase in chloride ion concentration that is a necessary consequence of equation [6], a whole series of copper chloride species becomes possible in the burial environment, where further reaction with copper or copper corrosion products could lead to the formation of complex species such as CuCl2, CuCl3−, CuCl42−. Indeed, Fabrizi and Scott (1987) found crystalline eriochalcite, CuCl2.2H2O, occurring as a corrosion product on a copper alloy object from Memphis, Egypt. This is a noteworthy occurrence, since high chloride ion contents of Egyptian soils can lead to the formation of unusual products. Eriochalcite may also be formed as a result of the alteration of nantokite in the laboratory. It could not, of course, survive in moist conditions since eriochalcite is soluble in water, but the site was a dry one and showed unusual corrosion products, such as sampleite, which is discussed in detail by Fabrizi et al. (1989). There is still much to be understood about the chemistry and pH conditions that prevail in copper objects and lead to an accumulation of cuprous chloride in the corrosion products. The cupric complexes must play an important role in the continued reactions giving rise to bronze disease before either all of the cuprous chloride is consumed or the humidity levels in pits or in zones contiguous with the surface drop below levels required for continuous reaction. |