AN INVESTIGATION OF THE REMOVABILITY OF NATURALLY AGED SYNTHETIC PICTURE VARNISHESSUZANNE QUILLEN LOMAX, & SARAH L. FISHER
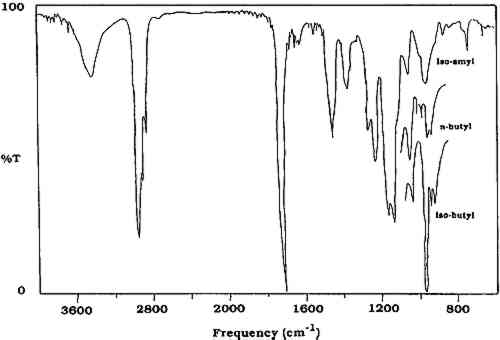
2 INFRARED SPECTROSCOPYIN THIS study, the analytical tool selected for identifying varnish removed from paintings was infrared spectroscopy. Pyrolysis gas chromatography has been used extensively to qualitatively and quantitatively examine acrylic polymers (Radell and Strutz 1959; Lehmann and Brauer 1961; Lehrle and Robb 1967; Esposito and Swann 1965; Ettre and Varadi 1962). Frankoski and Siggia (1972) have also examined alkali fusion reaction gas chromatography in the study of polyacrylates and polymethacrylates. Based on instrumentation available in our laboratory, however, and on ease of sample manipulation and analysis, infrared spectroscopy was employed to identify the synthetic varnishes removed from paintings. This technique allows for the analysis of organic molecules by identification of basic structural components. A Mattson Alpha Centauri Fourier Transform Infrared (FTIR) spectrometer was used for all analyses. The varnish material removed by swab was extracted with methylene chloride, and the resulting solution was concentrated and spotted on a KBr pellet for infrared analysis. This FTIR technique allows the detection of traces of resins on the swab that could not be visually detected. A literature search of the Mellon Institute Quarterly Reports(Feller 1956) indicated that standard aged varnish samples had been placed above the laylights of the National Gallery of Art in 1956. This is an uninsulated area between the interior skylights of the galleries and the roof of the building. Although no specific aging conditions were reported for these samples, they were exposed to both unfiltered daylight and extremes of temperature. It was roughly estimated (Feller 1959) that exposure in the “attic,” above the laylights, is about 15 times that in the galleries. Eleven of these aged samples were available. In order to supplement the collection of naturally aged materials, contact was made with Marjorie B. Cohn, former head conservator at the Center for Conservation and Technical Studies, Fogg Art Museum. Permission was given for the collection there to be sampled, and more than 150 naturally aged samples dating from 1933 to 1946, including natural and synthetic resins, oil-varnish mixtures, and other media, were collected. In order to have additional reference materials, samples of natural and synthetic coatings for artificial (thermal) and natural aging were prepared at the National Gallery of Art. Glass panels or slides were coated with films (thickness not measured) of various varnishes. Each sample was prepared in duplicate. One series was placed in a dry circulating oven at 100�C for more than one year, and the other was placed against a wall in the laboratory exposed to fluorescent lights for nine hours a day for more than one year. Samples from the oven-aged panels were used to compare changes in fluorescence, solubility, and infrared spectra upon aging. The samples designated for natural aging were used as controls. All of these unaged, naturally aged, and artificially aged varnishes of known composition were examined by infrared spectroscopy to obtain a data base against which unknowns could be compared. Aged samples of the natural resins dammar and mastic are too similar to be distinguished by infrared spectroscopy. Infrared spectra of the naturally aged (Fogg) and oven-aged samples of these two natural resins show virtually the same absorbances and intensities. Both of these materials have carbonyl (C=O) absorbances in the region of 1715–1700 cm−1, as well as multiple absorbances in the region 1270–1000 cm−1. Polycyclohexanone resins such as Laropal K-80 and AW-2 (BASF) also have carbonyl absorption in the region 1715–1700 cm−1, as does Rembrandt varnish (Talens). However, these materials are characterized by lack of an absorbance in the region of 1380 cm−1, which is present in dammar and mastic, as well as by the presence of a strong absorption at 1060 cm−1. Hydrogenated polycyclohexanone such as MS-2A possesses a strong absorbance at 3420 cm−1. The methacrylate polymers and other synthetic polymers have carbonyl absorbances in the region of 1740–1730 cm−1(Sadtler Research Laboratories 1980). The infrared spectra of these methacrylate polymers do not change substantially upon aging. A slight loss of resolution is often observed, but the absorbances and intensities remain constant. The methacrylate polymers can be readily distinguished from poly(vinyl acetate) because the latter possesses one strong absorbance at 1240 cm−1(table 1). The methacrylate polymers have a weaker double band at 1269 and 1241 cm−1. In addition, the region from 1175 to 970 cm−1 is different for the two classes of polymers. Poly(vinyl alcohol) is easily identified by the strong O-H absorbance at 3350 cm−1(Thomson 1963). TABLE 1 Selected Infrared Absorbances of Natural and Synthetic Varnishes (in cm−1) Distinguishing between the methacrylate polymers can often be difficult, especially if the sample is small or is “contaminated” with other varnishes. Fortunately, n-butyl, isobutyl, and isoamyl methacrylate homopolymers can be distinguished by comparing the regions from 1000–950 cm−1. As shown in figure 1, the spectrum of the n-butyl polymer consists of two doublets, while the isobutyl polymer has a triplet, with one of the three peaks being much more intense than the other two. The spectrum of the isoamyl polymer is characterized by a stronger single absorbance at 970 cm−1 with a small peak at 1130 cm−1. Therefore, it usually is possible to distinguish between these three methacrylate polymers by infrared spectroscopy, provided that the spectrum is sufficiently resolved in the region of 1000 to 950 cm−1. Usually a small amount of poly(isobutyl methacrylate) is found as an impurity in poly(n-butyl methacrylate), and its presence can be detected in most cases in the infrared spectrum.
|